Journal of Neurology, Neurological Science and Disorders
Current Pathogenetic Concepts of Vascular Cognitive Impairment
Kurt A Jellinger*
Cite this as
Jellinger KA (2016) Current Pathogenetic Concepts of Vascular Cognitive Impairment. J Neurol Neurol Sci Disord 2(1): 010-016. DOI: 10.17352/jnnsd.000009The term vascular cognitive impairment designates a heterogenous group of disorders ranging from mild cognitive impairment to full-blown dementia - vascular dementia - resulting from cerebrovascular lesions involving various brain areas. Current clinical criteria show moderate sensitivity (50-56%) and variable specificity (range 64-98%). The prevalence in autopsy series ranges from 0.03 to 58% (mean 8-15% in Western series, 22-35% in Japan). Major morphological types - multi-infarct and subcortical vascular encephalopathy, strategic infarct dementia, lacunar state, cortical granular atrophy (rare), and ischemic encephalopathy - are caused by atherosclerosis of major cerebral arteries and small vessel disease, resulting from systemic, cardiac and local vascular disease or cerebral amyloid angiopathy. Pathogenesis of vascular dementia is multifactorial, and pathophysiology affects brain areas and neurological networks involved in cognition, memory, behavior, and executive functions. Vascular brain injury in elderly persons often coexists with Alzheimer-type lesions and other pathologies resulting in mixed dementia. However, these lesions are also present in many non- demented elderly subjects. The heterogeneity of clinical manifestations, cerebrovascular pathology and their pathogenic factors result in limitations of the accuracy of diagnostic criteria for vascular dementia. Therefore, standardized and reproducible neuropathological criteria for the assessment of cerebrovascular lesions associated with cognitive impairment in order to elucidate contribution of cerebrovascular disease to cognitive impairment are urgently needed.
Abbreviations
AD: Alzheimer Disease; BBB: Blood-Brain Barrier; CAA: Cerebral Amyloid Angiopathy; CFS: Cerebrospinal Fluid; CMB: Cerebral Microbleed; CMI: Cerebral Microinfarct; CVD: Cerebrovascular Disease; CVL: Cerebrovascular Lesion; MCI: mild cognitive impairment; MIE: Multi-Infarct Encephalopathy; MRI: Magnetic Resonance Imaging; SVD: Small Vessel Disease; VaD:Vascular Dementia; VCD: Vascular Cognitive Disorder; VCI: Vascular Cognitive Impairment; VCIND: Vascular Cognitive Impairment No Dementia; WMH: White Matter Hyperintensity; WML: White Matter Lesion
Introduction
Cerebrovascular disease (CVD) is increasingly recognized as a major cause of cognitive impairment in later life either alone or in conjunction with AD or other pathologies [1]. Vascular cognitive impairment (VCI) [2], first described as “arteriosclerotic dementia”, comprises a hetereogenous group of cognitive disorders of various severity and types ranging from subtle deficits to full-blown vascular dementia (VaD) that share a presumed vascular etiology. Vascular cognitive disorder (VCD) represents a global diagnostic category encompassing all these disorders [3], while subcortical vascular dementia designates a more hetereogenous syndrome [4]. The clinical diagnosis, epidemiology, pathophysiological and pathogenic concepts of VCI/VaD are in the focus of current neuroscience research [2,5].
Clinical features
Cognitive changes in VCI/VaD are more variable than in AD and are dependent on the particular neuronal substrates related to the vascular pathology, while other functions such as memory, language, executive functions and non-cognitive features, depression and apathy are much more affected; delusions and hallucinations are less frequent [6,7].
Previous diagnostic criteria for VCI/VaD requested the presence of memory loss independent of dementia. Currently, several sets of criteria for the clinical diagnosis of VCI/VaD are used (see [8]): the NINDS-AIREN criteria [9], the ADDTC criteria [10], the DSM-V criteria (APA), distinguishing possible, probable and proven VaD (with pathologically proven multiple cerebrovascular lesions/CVLs) [11]; the NINDS-CSN criteria [12], the EFNS guidelines [13], the consensus statement of the American Stroke Association [14], and the VASCOG criteria [6].
Vascular cognitive impairment no dementia (VCIND) describes a condition related to vascular disease, in which cognitive impairment is not severe enough to fit the criteria of dementia [15].
Several studies reported moderate sensitivity of clinical criteria (average 50-56%) and variable specificity (range 64-98%) with variable interrater reliability [7,16]. The limitations of current clinical diagnostic criteria sets for VCI/VaD that poorly reflect the underlying pathology, have been critically discussed recently [6,17].
In clinical studies, the prevalence of VaD ranges from 4.5 to 39%, in Western memory clinic- and population-based series 8-15%, in pathological series even 0.03-85.2% with means around 11-15%, and in Japan 23.6-35% [8]. The prevalence studies must be interpreted cautiously since aged subjects with and without dementia show a high frequency of mixed pathologies [18-22].
In elderly patients the prevalence of “pure” VaD morphologically characterized by multiple CVLs without essential concomitant AD-type (Braak neuritic stage < 2.0) and other pathologies ranges from 5 to 78% with mild reduction in the oldest-old, while that of mixed dementia increases with age [23]. Recent studies have emphasized the co-morbidities associated with VaD and AD-like pathology. Although there is an established relationship between vascular and degenerative (AD) pathology, the mechanistic links between the two have to be identified. In general, however, vascular brain damage is believed to be an important component of AD pathophysiology [24]. Just one example: Analysis of 4629 cases of the NACC database with autopsy-confirmed AD classified 79.7% as having additional CVD [25]. However, the impact of co-occurring pathologies on progression of cognitive impairment may depend on the severity of AD pathology [1,21,26].
Pathology of VCI/VaD
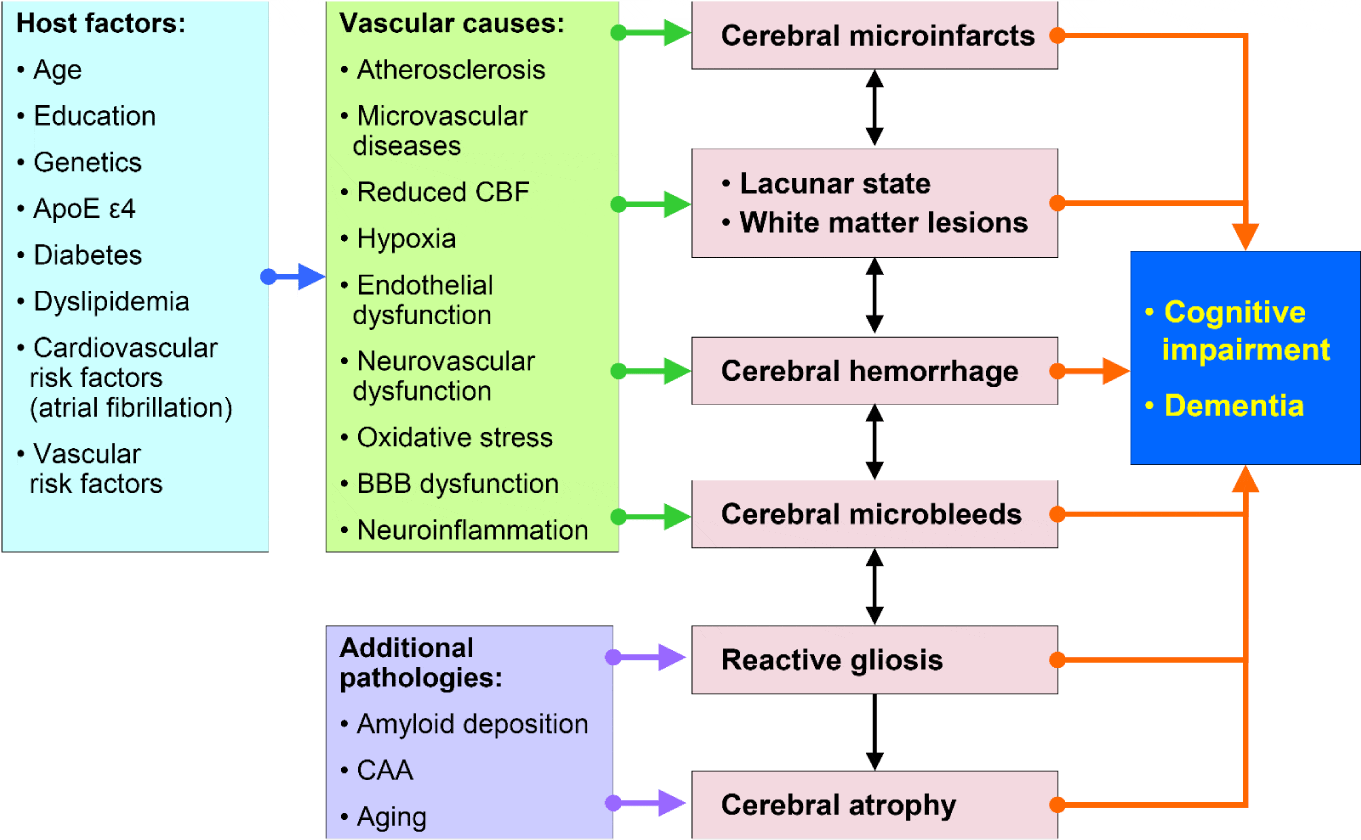
Pathological changes in the brains of patients with cognitive impairment are highly variable and multifold including focal, multifocal and diffuse lesions (Table 1). They are related to large and small vessel disease.
1. Large vessel dementia: Multi-infarct encephalopathy (about 15% of VCI) is featured by multiple large and small infarcts involving the supply areas or borderzones of major cerebral arteries due to severe atherosclerosis of extra- and intracranial vessels giving rise to local thrombo-embolism and hypoperfusion, while inflammatory angiopathies and rare hereditary forms, e.g., CADASIL, more frequently cause smaller infarcts. Arterial embolism is caused by breaking of thrombi from ulcerated lesion in extracranial arteries or heart valves.
2. Small vessel disease (SVD)/microangiopathic dementia with lacunes, microinfarcts and microbleeds, predominantly involving subcortical structures - white matter, basal ganglia, intern capsule - due to microvascular changes (fibrosis, stenosis, hypertensive angiopathy, etc.) being present in more than 60% of VCI patients. Lacunar infarcts are miliary softenings from 3 to 15 mm diameter, found in 32-45% of elderly as the most frequent types of CVLs [17]. They are common and strongly related to dementia in the oldest-old [27].
Small vessel lesions which are seen by modern neuroimaging methods (particularly 7-Tesla MRI) [28] can be distinguished according to the type and predominant location:
a) (Multi) lacunar state with multiple cortico-subcortical lacunes or microinfarcts.
b) Strategic infarct dementia (SID) with small infarcts in functionally important brain areas (thalamus, frontocingular cortex, hippocampus) due to SVD or embolism.
c) Watershed or borderzone (cortical and/or subcortical) infarcts in borderzones between major cerebral arteries or in territories between small deep and superficial vessels (hippocampal, thalamic infarcts) caused by diminished blood flow due to atherosclerosis or prolonged hypotension.
d) Subcortical vascular dementia, arteriosclerotic (leuko) encephalopathy (Binswanger disease) or leukoaraiosis with confluent white matter lesions (demyelination, axonal loss due to lacunar infarcts or related with enlarged perivascular areas), usually sparing subcortical U-fibers. White matter hyperintensities (WMH), seen in magnetic resonance imaging (MRI) in elderly individuals with and without cognitive impairment, are generally associated with SVD and edema, but recent studies indicate also relationship to cortical tau pathology in Alzheimer disease (AD) [29]. The lesions including demyelination, axonal loss, lacunar infarcts or dilatation of the Virchow-Robin spaces are caused by disturbances of the blood-brain barrier (BBB), hypotension and chronic ischemia. Identification of this subgroup can be facilitated by a set of multimodal disease markers, obtained from clinical, cerebrospinal fluid, neuropsychological, and imaging studies [30].
e) Cerebral microinfarcts (CMIs), detected by 3T and 7T MRI [28,31] involving subcortical areas are associated with atherosclerosis and arteriolosclerosis, while cortical microinfarcts are mainly associated with cerebral amyloid angiopathy (CAA) [32]. The etiology of cerebral microinfarcts differs depending on brain region. CAA is important in the occipital lobe, while in frontal cortex and hippocampus CMIs without any vascular pathology were significantly more frequent, indicating that hypoperfusion or large vessel atherosclerosis may play a role in the development of microvascular lesions in these areas [33].
f) Cerebral microbleeds (CMBs), involving both cortex and white matter, as detected by MRI [28], are caused by hypertensive SVD and CAA [34]. Cortical microbleeds are often associated with CAA, whereas subcortical ones are mainly related to SVD or embolies, but both subcortical SVD and CAA interact to increase the risk or lobar CMBs [35]. They are associated with cognitive decline [36], although their mechanisms and their interaction with cognitive impairment remain uncertain [26]. Brains without significant AD pathology showed more severe lacunes and microinfarcts than subjects with large infarcts and those without dementia [37].
3. Other pathological substrates of VCI include:
a) Post-ischemic encephalopathy with several types of predominant distribution pattern: (1) cortical laminar necrosis often involving arterial border zones and associated with white matter damage resulting from hypoxia due to cardiac or respiratory arrest; (2) multiple post-ischemic lesions with cortical/subcortical (micro)infarcts after cardiac arrest and cerebral atherosclerosis; (3) hippocampal sclerosis of hypoxic-ischemic etiology, relatively common (ca 10%) associated with dementia in very old patients but also associated with a variety of neurodegenerative disorders, such as frontotemporal lobar degeneration and TDP-43 pathology [38,39].
b) Hemorrhagic dementia with primary (hypertensive) intracerebral bleeds due to hypertension is rare. Cognitive dysfunctions related to multiple cortical and subcortical hemorrhages usually are associated with sporadic or hereditary CAA, although the majority of CAA-related cerebral hemorrhages had hypertension, suggesting it is an important additonal causal factor in CAA-related intracerebral hemorrhages [40].
c) Combined (multifocal) CVLs involving various brain regions, related to different vascular dysfunctions [23], including hereditary SVD, e.g., CADASIL or CARASIL [41].
As for other pathologies underlying CVI/VaD, there is basal consensus that it results from brain dysfunction caused by cumulative tissue damage [14,17,23,42].
Recent neurophysiological studies using transcranial motor stimulation (TMS) have revealed distinct patterns of cortical excitability in VaD, possibly associated with disease process and progress [43,44]. However, the pathophysiological details of VCI/VaD and of vascular depression (VaDep - see consensus paper [45]) are beyond the scope of this mini-review.
Risk factors for CVI/VaD
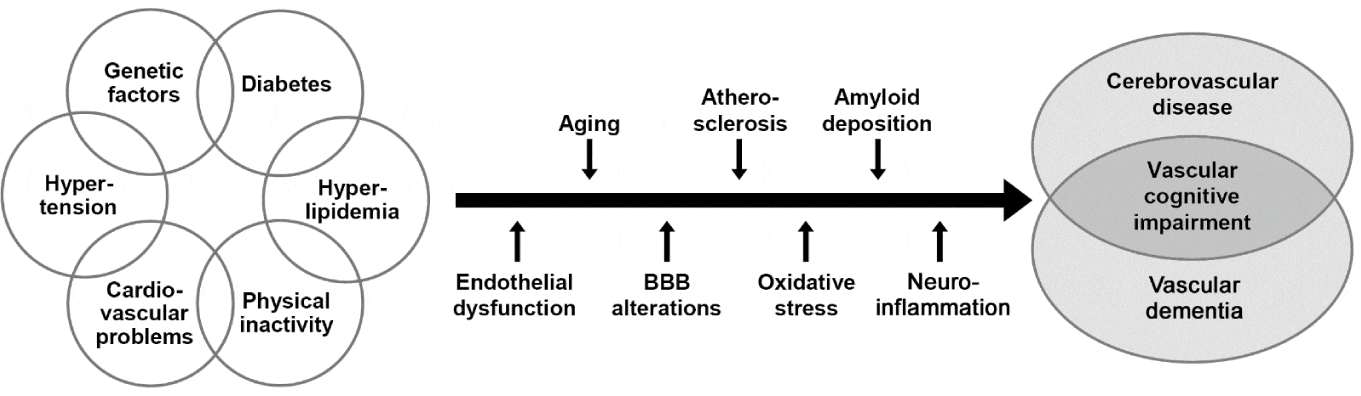
Risk factors for VCI/VaD are multifold and include age, midlife hypertension, dyslipidemia, diabetes mellitus, cardiovascular and lifestyle factors, arterial disease, recurrent stroke, asymptomatic vascular brain injury (silent stroke), smoking, low education, physical inactivity [14,46], while others suggested hypotension to be such a risk factor [47]. Many of these and other other risk factors like late life depression [45] are common to VaD, AD and mixed dementia. Potential mechanisms between risk factors, CVD and cognitive impairment are given in Figure 1. The detrimental effects of social isolation on CVDs and related cognitive disorders have been discussed recently [49], and the role of vascular depression as a subtype of late-life depression and its relations to cerebrovascular disorders has been reviewed in a recent consensus report [45,50]. Psychosocial stressors have been shown to exacerbate disease-related morbidity and mortality [51].
Pathogenesis of CVI/VaD
The pathogenesis of CVLs inducing cognitive impairment is multifactorial, depending on origin, volume and location of brain destructions and the distribution, number and severity of CVLs. Neuroimaging and clinico-pathologic studies have clearly indicated that the threshold for VCI/VaD depends on the extent and location of cerebral damage [17,26,29]. Chronic cerebral hypoperfusion is a common pathophysiological condition frequently occurring in VaD. Within CVD, the most common contribution is likely central SVD, which involves tissue injury in both cortical and subcortical gray and white matter, but may coexist with atherosclerosis involving large extracranial vessels and embolic disease [4,17,41]. Vessel wall changes such as arteriolosclerosis and CAA were proposed to be the most earliest and common changes, followed by perivascular spacing with lacunar and regional microinfarcts progressing from frontal to temporal lobe and basal ganglia [52].
The molecular mechanisms active in VaD and shared among almost all the dementia types include neuronal damage, BBB alterations, hypoxia, oxidative and nitrosative stress, mitochondrial dysfunction, autophagy, neuroinflammation, neurodegeneration, etc., due to reduced brain perfusion, which contribute to and exacerbate the etiology and course of the disease [51,53]. These molecular links between VaD and AD have been discussed recently [54].
The resulting lesions involve various, functionally important brain areas (prefrontal cortex, thalamus, hippocampus, etc.) and neuronal networks with deafferentation of frontal and limbic structures and damage to neuronal networks important for cognition, memory, behavior and executive functions [23]. Disruption of subcortico-frontal circuits due to white matter lesions by subcortical microinfarcts resulting in cognitive impairment has been confirmed by experimental models of cerebral ischemia [55].
The neurochemical basis of CVD is poorly understood. Diffuse WMLs involving the basal forebrain cholinergic system cause widespread disconnection of cholinergic projections [56], indicating an association of cholinergic dysfunction with dementia severity in subcortical VCI [57]. Loss of cholinergic function is greater in VaD with concurrent AD pathology [58,59], whereas central cholinergic pathways do not seem involved in VCIND, thus differing from primary cholinergic forms of dementia, such as AD [60]. Others have reported deficits in monoamines including dopamine and serotonin in the basal ganglia and neocortex [61] and of presynaptic proteins in the temporal cortex in VaD [62].
Although there is a frequent overlap between vascular and degenerative lesions causing cognitive impairment [17,24], and a frequent lack of correlations between clinical and pathology findings [26], there is a consistent interplay of pathogenic factors causing VCI (Figure 2). Due to frequent co-morbidity in old age, cerebrovascular pathology often coexists with Alzheimer-type lesions and other pathologies. 25 to over 80% (mean 75%) of elderly both demented and non-demented individuals show mixed pathologies [21,24,42].
New classification proposals
Neuropathology has to describe the nature, severity and localization of CVLs related to cognitive decline using harmonized procedures and criteria [63], addressing the question whether the vascular lesions present in a particular brain are of sufficient magnitude and relevant location to contribute to cognitive impairment.
Standardized neuropathological criteria for the assessment of CVLs associated with cognitive impairment are urgently needed. However, despite several recent suggestions for staging and grading vascular lesions in specific brain areas, due to the high variability of these lesions, no generally accepted and validated neuropathological criteria were until recently available for VCI/VaD [23].
There are several international efforts to define cognitive impairment due to cerebrovascular disease in different stages and subtypes: (1) The Newcastle group suggested a categorization of the cerebrovascular pathology associated with cognitive impairment according to 6 subtyes of CVLs ranging from large or several infarcts, multiple microinfarcts, infarcts in strategic areas, cerebral hypoperfusion, hemorrhages, and cerebrovascular changes associated with AD-negative pathology [64]; (2) the revised NIA-AA guidelines recommended to report macroscopic vascular brain injuries and microvascular impairment [65], and (3) staging of CVLs using a semiquantitative assessment in four brain areas with a score ranging from I to IV/VI [52]. All these proposals, however, await further validation by clinico-pathological studies.
A recent collaborative study of nine United Kingdom neuropathological centers to formulate evidence-based Vascular Cognitive Impairment Neuropathology Guidelines (VCING) for post-mortem assessment of CVD of relevance for VCI/VaD has shown that various combinations of 3 pathologies (occipital leptomeningeal CAA, atherosclerosis in occipital white matter, and at least one infarct) can be used to report a low, intermediate or high likelihood that CVD contributed to cognitive impairment [66]. Like previous proposals for classification and rating of vascular and related cerebral lesions causing cognitive impairment, the VCING needs validation. A recent consensus report of the International Congress on Vascular Dementia working group discussed whether the time is ripe for new diagnostic criteria of cognitive impairment due to CVD [67].
Conclusions and Further Outlook
Modern neuroimaging together with clinical and biological marker studies will continue to play a leading role in the diagnosis of dementias, in particular a more clear delineation between VCI/VaD, AD, and mixed pathology [26,28]. However, neuropathology still remains the “gold standard” of diagnosis. The preclinical stages of VCI have to be defined more precisely and the term VCI should be used in a similar way as MCI due to AD to characterize the early clinical stages of VCI [67]. In addition, more precise relations between clinical and neuroimaging longitudinal findings with postmortem changes should be performed.
Defining neuropathological substrates of VCI/VaD relies on uniformity in sampling and careful pathological examination. While robust clinical and neuropathological diagnostic criteria for VCI/VaD are still being developed, multiple microinfarcts or lacunes in subcortical structures and deep white matter due to widespread SVD rather than macroinfarction or large vessel disease appear most robustly related to cognitive impairment. It should be considered that concomitant AD-like lesions and hippocampal pathology including sclerosis induce disease progression. Further clinico-pathological studies and harmonization of neuropathological procedures are needed to validate the diagnostic criteria for VCI/VaD in order to elucidate the impact of CVLs and coexistent pathologies on cognitive impairment as a basis for successful preventive and treatment options.
- Jellinger KA, Attems J (2015) Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm (Vienna) 122: 505-521. Link: https://goo.gl/73cDob
- Rincon F, Wright CB (2013) Vascular cognitive impairment. Curr Opin Neurol 26: 29-36. Link: https://goo.gl/Mm3PKg
- Román GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, et al. (2004) Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci 226: 81-87. Link: https://goo.gl/Jtnvor
- Rincon F, Wright CB (2014) Current pathophysiological concepts in cerebral small vessel disease. Front Aging Neurosci 6: 24. Link: https://goo.gl/76opKh
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, et al. (2016) The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol 36: 281-288. Link: https://goo.gl/obcNt6
- Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, et al. (2014) Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis Assoc Disord 28: 206-218. Link: https://goo.gl/qHABrv
- O'Brien JT, Thomas A (2015) Vascular dementia. Lancet 386: 1698-1706. Link: https://goo.gl/Ys3V2Q
- Jellinger KA (2014) Pathogenesis and treatment of vascular cognitive impairment. Neurodegener Dis Manag 4: 471-490. Link: https://goo.gl/AXACdb
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, et al. (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: 250-260. Link: https://goo.gl/CVV6Li
- Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, et al. (2000) Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Arch Neurol 57: 191-196. Link: https://goo.gl/sj66UF
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-V). Arlington, VA: American Psychiatric Association. Link: https://goo.gl/CWLpFF
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, et al. (2006) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37: 2220-2241. Link: https://goo.gl/hYKseU
- Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, et al. (2012) EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol 19: 1159-1179. Link: https://goo.gl/w8Sa3K
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, et al. (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42: 2672-2713. Link: https://goo.gl/rbsGBs
- Stephan BC, Matthews FE, Khaw KT, Dufouil C, Brayne C (2009) Beyond mild cognitive impairment: vascular cognitive impairment, no dementia (VCIND). Alzheimers Res Ther 1: 4. Link: https://goo.gl/ThQkC3
- Bacchetta JP, Kovari E, Merlo M, Canuto A, Herrmann FR, et al. (2007) Validation of clinical criteria for possible vascular dementia in the oldest-old. Neurobiol Aging 28: 579-585. Link:
- Kalaria RN (2016) Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathol 131: 659-685. Link: https://goo.gl/NhfknA
- Jellinger KA (2009) Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp? Acta Neuropathol 117: 101-110. Link: https://goo.gl/lLfp40
- Ferrer I (2010) Cognitive impairment of vascular origin: neuropathology of cognitive impairment of vascular origin. J Neurol Sci 299: 139-149. Link: https://goo.gl/cbJ7RE
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, et al. (2015) Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology 85: 535-542. Link: https://goo.gl/fmFDoU
- Brenowitz WD, Hubbard RA, Keene CD, Hawes SE, Longstreth WT, Jr., et al. (2016) Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement pii: S1552-5260(16)33057-6. Link: https://goo.gl/FKJ1Jy
- Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, et al. (2009) Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge City over-75s Cohort (CC75C) Study. J Alzheimers Dis 18: 645-658. Link: https://goo.gl/kSAEMA
- Jellinger KA (2013) Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci 5: 17. Link: https://goo.gl/pCLRpo
- Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer's disease - lessons from pathology. BMC Med 12: 206. Link: https://goo.gl/8OL6mi
- Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE (2013) Vascular disease and dementias: Paradigm shifts to drive research in new directions. Alzheimers Dement 9: 76-92. Link: https://goo.gl/ckLVUp
- McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, et al. (2016) Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med 14: 129. Link: https://goo.gl/h3lJpi
- Corrada MM, Sonnen JA, Kim RC, Kawas CH (2016) Microinfarcts are common and strongly related to dementia in the oldest-old: The 90+ study. Alzheimers Dement 12: 900-908. Link: https://goo.gl/RiaIfV
- Heiss WD, Rosenberg GA, Thiel A, Berlot R, de Reuck J (2016) Neuroimaging in vascular cognitive impairment: a state-of-the-art review. BMC Med 14: 174. Link: https://goo.gl/p9G0S3
- McAleese KE, Firbank M, Dey M, Colloby SJ, Walker L, et al. (2015) Cortical tau load is associated with white matter hyperintensities. Acta Neuropathol Commun 3: 60. Link: https://goo.gl/4hrKRb
- Rosenberg GA, Wallin A, Wardlaw JM, Markus HS, Montaner J, et al. (2016) Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 36: 6-25. Link: https://goo.gl/Edhj7X
- van Veluw SJ, Zwanenburg JJ, Rozemuller AJ, Luijten PR, Spliet WG, et al. (2015) The spectrum of MR detectable cortical microinfarcts: a classification study with 7-tesla postmortem MRI and histopathology. J Cereb Blood Flow Metab 35: 676-683. Link: https://goo.gl/dCaEgM
- Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, et al. (2016) The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol [Epub ahead of print] Link: https://goo.gl/w12Ez8
- Kovari E, Herrmann FR, Gold G, Hof PR, Charidimou A (2016) Association of cortical microinfarcts and cerebral small vessel pathology in the ageing brain. Neuropathol Appl Neurobiol [Epub ahead of print] Link: https://goo.gl/bKVXgY
- Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, et al. (2016) Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 73: 934-943. Link: https://goo.gl/ni3YYl
- Park JH, Seo SW, Kim C, Kim GH, Noh HJ, et al. (2013) Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol 73: 584-593. Link: https://goo.gl/teuju9
- Charidimou A, Werring DJ (2012) Cerebral microbleeds and cognition in cerebrovascular disease: An update. J Neurol Sci 322: 50-55. Link: https://goo.gl/PDdt4U
- Smallwood A, Oulhaj A, Joachim C, Christie S, Sloan C, et al. (2012) Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol 38: 337-343. Link: https://goo.gl/APJKxY
- Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, et al. (2013) TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 70: 1418-1424. Link: https://goo.gl/jT0p26
- Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, et al. (2013) Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 126: 161-177. Link: https://goo.gl/02Zhx0
- Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, et al. (2011) Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain 134: 2376-2386. Link: https://goo.gl/4AkhbO
- Tikka S, Baumann M, Siitonen M, Pasanen P, Poyhonen M, et al. (2014) CADASIL and CARASIL. Brain Pathol 24: 525-544. Link: https://goo.gl/LGkLIr
- Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80: 844-866. Link: https://goo.gl/4ZuSY1
- Bella R, Ferri R, Cantone M, Pennisi M, Lanza G, et al. (2011) Motor cortex excitability in vascular depression. Int J Psychophysiol 82: 248-253. Link: https://goo.gl/MqKR5e
- Lanza G, Bella R, Giuffrida S, Cantone M, Pennisi G, et al. (2013) Preserved transcallosal inhibition to transcranial magnetic stimulation in nondemented elderly patients with leukoaraiosis. Biomed Res Int 2013: 351680. Link: https://goo.gl/P61vZY
- Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, et al. (2016) Vascular depression consensus report - a critical update. BMC Med 14: 161. Link: https://goo.gl/AWI1kK
- Wiesmann M, Kiliaan AJ, Claassen JA (2013) Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab 33: 1696-1706. Link: https://goo.gl/6xh5zw
- Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, et al. (2008) Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag 4: 395-402. Link: https://goo.gl/9ZwUsI
- Levine DA, Langa KM (2011) Vascular cognitive impairment: disease mechanisms and therapeutic implications. Neurotherapeutics 8: 361-373. Link: https://goo.gl/ZMb5x5
- Friedler B, Crapser J, McCullough L (2015) One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol 129: 493-509. Link: https://goo.gl/znjUAQ
- Jellinger KA (2016) The enigma of vascular depression. Austin Alzh Parkinson Dis 3: 1026. Link: https://goo.gl/eY5vMx
- Calabrese V, Giordano J, Signorile A, Laura Ontario M, Castorina S, et al. (2016) Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J Neurosci Res 94: 1588-1603. Link: https://goo.gl/wykUsm
- Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, et al. (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78: 1043-1050. Link: https://goo.gl/ReROpG
- Raz L, Knoefel J, Bhaskar K (2016) The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab 36: 172-186. Link: https://goo.gl/No7o4a
- Vijayan M, Reddy PH (2016) Stroke, Vascular Dementia, and Alzheimer's Disease: Molecular Links. J Alzheimers Dis 54: 427-443. Link: https://goo.gl/Wtdc6K
- Sarti C, Pantoni L, Bartolini L, Inzitari D (2002) Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. J Neurol Sci 203-204: 263-266. Link: https://goo.gl/QVh3NV
- Swartz RH, Sahlas DJ, Black SE (2003) Strategic involvement of cholinergic pathways and executive dysfunction: Does location of white matter signal hyperintensities matter? J Stroke Cerebrovasc Dis 12: 29-36. Link: https://goo.gl/lnQplM
- Kim SH, Kang HS, Kim HJ, Moon Y, Ryu HJ, et al. (2012) The effect of ischemic cholinergic damage on cognition in patients with subcortical vascular cognitive impairment. J Geriatr Psychiatry Neurol 25: 122-127. Link: https://goo.gl/gwuXGd
- Román GC, Kalaria RN (2006) Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging 27: 1769-1785. Link: https://goo.gl/ycyn31
- Sharp SI, Francis PT, Elliott MS, Kalaria RN, Bajic N, et al. (2009) Choline acetyltransferase activity in vascular dementia and stroke. Dement Geriatr Cogn Disord 28: 233-238. Link: https://goo.gl/Zn2dJS
- Bella R, Cantone M, Lanza G, Ferri R, Vinciguerra L, et al. (2016) Cholinergic circuitry functioning in patients with vascular cognitive impairment--no dementia. Brain Stimul 9: 225-233. Link: https://goo.gl/hEvFRH
- Sinclair LI, Tayler HM, Love S (2015) Synaptic protein levels altered in vascular dementia. Neuropathol Appl Neurobiol 41: 533-543. Link: https://goo.gl/wLneBz
- Gottfries CG, Blennow K, Karlsson I, Wallin A (1994) The neurochemistry of vascular dementia. Dementia 5: 163-167. Link: https://goo.gl/HdleyD
- Alafuzoff I, Gelpi E, Al-Sarraj S, Arzberger T, Attems J, et al. (2012) The need to unify neuropathological assessments of vascular alterations in the ageing brain: multicentre survey by the BrainNet Europe consortium. Exp Gerontol 47: 825-833. Link: https://goo.gl/QQRlWS
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, et al. (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226: 75-80. Link: https://goo.gl/HGZRyb
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 123: 1-11. Link: https://goo.gl/5IXWjI
- Skrobot OA, Attems J, Esiri M, Hortobagyi T, Ironside JW, et al. (2016) Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain in print Link: https://goo.gl/3QXFpJ
- Perneczky R, Tene O, Attems J, Giannakopoulos P, Ikram MA, et al. (2016) Is the time ripe for new diagnostic criteria of cognitive impairment due to cerebrovascular disease? Consensus report of the International Congress on Vascular Dementia working group. BMC Med 14: 162. Link: https://goo.gl/ikFQxo
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
