Journal of Neurology, Neurological Science and Disorders
Comparing Staircase and Skilled Forelimb Reaching Tests After Endothelin-1-Induced Stroke
Lindsey D Jager, Claire-Marie A Canda, Megan L Gilbertson, Crystal A Hall, Cassandra L Heilingoetter, Joann Huynh, Susanna S Kwok, Jin H Kwon, Jacob R Richie, Natanya S Russek and Matthew B Jensen*
Cite this as
Jager LD, Canda CMA, Gilbertson ML, Hall CA, Heilingoetter CL, et al. (2017) Comparing Staircase and Skilled Forelimb Reaching Tests After Endothelin-1-Induced Stroke. J Neurol Neurol Sci Disord 3(1): 016-022. DOI: 10.17352/jnnsd.000014Background: Stroke is a leading cause of death and disability worldwide, but there are limited treatment options available despite extensive animal studies. A possible contributing factor for this discrepancy involves limitations of behavioral testing outcomes to assess therapeutic interventions. There are few comparisons to aid the researcher in choosing the most appropriate test. To address this issue, we compared the ability of two of the most commonly used fine-motor skills tests, the Montoya staircase and skilled forelimb reaching tests, to detect endothelin-1-induced ischemic strokes in the rat.
Methods: Rats were trained for 2 weeks followed by 2 weeks of testing to establish baseline behavior. Rats then received intracerebral injections of increasing volumes of endothelin-1 to induce ischemia. 7 days post-injury, rats were re-tested. Stroke volume was estimated on brain sections.
Results: 8 uL of endothelin-1 produced larger strokes than 2 or 4 uL injections. The skilled forelimb reaching test was able to detect post-stroke deficits in 4 and 8 uL rats while the staircase test only detected deficits in the 8 uL rats.
Conclusion: The skilled forelimb reaching test may be more appropriate for researchers studying the effects of smaller strokes.
Introduction
In the era of modern scientific research, animal subjects have proven to be vital to virtually every advance; yet to date there has only been one medical intervention for ischemic stroke approved by the Food and Drug Administration, recombinant tissue plasminogen activator [1,2]. Many reasons have been broached for this discrepancy, including the inherent physiological differences between animals and humans, poorly designed studies introducing bias which overestimate the therapeutic potential of the intervention being tested, the focus on histological rather than functional outcomes such as infarct volume, and the use of young, otherwise healthy animals lacking the cerebrovascular comorbidities so often found in human patients [1-4].
Another possible reason for the lack of clinical implication for many preclinical studies is the sheer variety of injury models being used and outcomes being assessed, creating a barrier to comparing results [3]. As Fisher suggests, the “ultimate goal is to show that in concert with these target and tissue endpoints, functional neurological outcomes are also improved in treated populations” [2]. To achieve this, animal stroke models must demonstrate clear and appropriate functional and behavioral deficits after infarct and display recovery after therapeutic intervention. Therefore, it is essential to perform the most responsive behavioral tests to yield the most accurate results [5]. Yet with many labs creating their own functional tests to suit their own needs, assets, and funding environments, it can be difficult to choose the best test for the job, particularly since novel tests are often used without simultaneous comparisons to previously published tests [3].
Loss of motor function is one of the most common pathologies following stroke, and can have a profound effect on the ability of patients to comfortably and competently participate in daily life [6]. Lacunar strokes make up 25% of all ischemic strokes, and the most common of the lacunar syndromes is the pure motor stroke [7]. Steinke and Ley showed that lacunar strokes are a major source of motor deficits [8]. Impairment of the fine motor skills of the hand can be particularly debilitating, and function is often not recovered in patients with severe paresis of the arm [6,9,10]. In a study of nearly 300 patients, Houwink, Nijland, and Geurts, et al., showed that less than half of those who began the study with no hand capacity exhibited improvement after rehabilitation [10].
Two of the most widely used behavioral tests in rodent models of stroke for addressing fine motor skills function and recovery include the Montoya staircase test and the skilled forelimb reaching task (also known as the single pellet reaching test) [11-13]. A brief search of PubMed using the terms stroke, rodent, staircase, and skilled reaching yields more than 500 results. Skilled reaching is considered to be a relevant test for studying pre- and post-injury fine motor skills because although there are differences between the species, the behavior in rats and humans uses similar movements and strategies for success [14-16].
In the Montoya staircase test, the animal must climb onto a counter and reach down to a series of steps, isolating the left front paw from the right [11,17,18]. On each step an incentive is placed and the number retrieved by each forepaw is recorded after a standard time period has elapsed. This test measures independent forelimb extending and grasping skill. While the test may be recorded for detailed kinematic analysis of the reach, it is most often employed as a quantitative test, reporting only the percent success rate [18,19]. In the skilled forelimb reaching test, the animal is placed in a box and learns, after extensive training, to extend its dominant forepaw through a vertical slit to retrieve a food item from a fixed location [12,14,17,18]. The rat is allowed to choose which paw it prefers to use, thus handedness must be taken into account when designing the study [13]. The number of reaches, successful retrievals, and failures is recorded. Additionally, the skilled forelimb reaching test is often used to qualitatively analyze the individual components of the reach, such as posture stabilization, arm lift and extension, the mechanics of the grasp itself, and release of the pellet into the mouth [14,18].
In order to mitigate time and material expenses, as well as potentially decrease the numbers of animals involved, labs should be encouraged to choose those tests which have been proven to work in their particular model. There is no standard animal model capable of mimicking all of the features of lacunar stroke, and many models that try end up producing cortical strokes instead [20]. Ours is the first study that directly compares the staircase and skilled reaching tests in a rat model following the induction of small ischemic strokes using the vasoconstrictor peptide endothelin-1 with the express purpose of determining which test is more efficient for studying this type of brain injury.
Methods
Animals
All procedures involving animals were approved by the University of Wisconsin-Madison Animal Care and Use Committee. All animals included in the study were adult (250-300g), male, Sprague-Dawley rats (Charles River, Wilmington, MA). Rats were housed in standard cages (Allentown Cages, Allentown, PA) on a 12-hour light/dark cycle and were fed Teklad Global 19% Protein Extruded Rodent Diet (Harlan Laboratories, Indianapolis, IN). There are 2 parts to this study. In part 1, 22 adult, male Sprague-Dawley rats were randomly assigned to receive 2, 4 or 8uL of the vasoconstrictor peptide endothelin-1 (ET-1, American Peptide), and underwent behavioral and histological analysis. Rats were randomly assigned to the groups using a random number generator (www.random.org). Three rats were removed from that portion of the study due to not surviving the surgery. In part 2, we sought to determine what, if any, effect handedness had on the behavioral tests using an additional 28 rats which all received 8uL ET-1.
Skilled reaching tasks
Rats were trained to reach for food rewards, mini-chocolate chips (Hershey’s, Hershey, PA) and 45mg sugar pellets (Bio-Serv, Flemington, NJ), in the Montoya Staircase Box and the single pellet reaching task, respectively. Animals were habituated to the food rewards by placing a sample of each in their home cages daily for 5 days prior to the onset of training. Training lasted 2 weeks and was performed 5 days/week, 10 minutes/day. Beginning 3 days before training, all rats were placed on a restricted diet (maintaining no less than 85% free-feed bodyweight) to increase motivation to perform behavioral tests. Separate food rewards were chosen for each task also for this reason. They stayed on this restricted diet for the remainder of the study, with the exception of the day of the surgical procedure when they were free-fed overnight. Testing lasted 2 weeks prior to stroke induction and was performed 5 days/week. Rats were then tested again 7 days after the stroke. Raters were blinded to which group the rats belonged.
Training-staircase
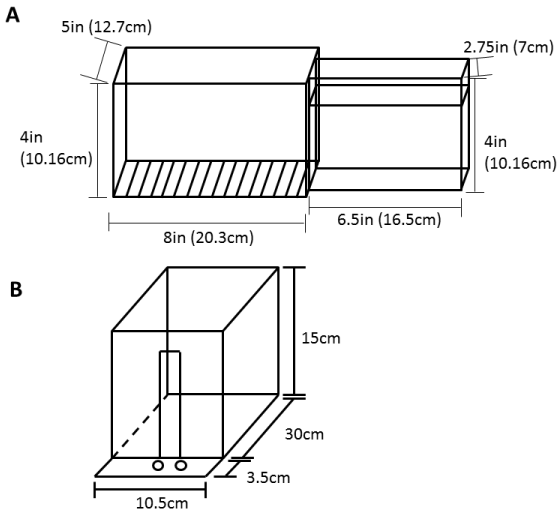
Rats were placed in a modified Montoya Staircase Box constructed of clear acrylic for 10 minutes per session (Figure 1a) [11]. The rat rests on a central elevated counter in the narrow forward chamber with eight steps descending on either side. Steps on the left can only be reached with the left paw and steps on the right can only be reached with the right paw, thus providing a measure of independent forelimb skilled reaching. A single mini-chocolate chip was placed on each step and re-baited as necessary to encourage participation during the session.
Training-skilled forelimb reaching
Rats were placed in a clear acrylic box with a 1cm wide slit opening onto a shelf containing divots to hold 45mg sugar pellets for 10 minutes per session (Figure 1b) [12,13]. The divots are placed lateral to the opening so that the rat cannot reach a pellet placed in the right divot with his right paw or a pellet placed in the left divot with his left paw. Several pellets were initially placed on the shelf to encourage the rat to reach through the slit (days 1-3 of training depending on the rat). Once a preference for handedness was established, the pellets were placed only in the divot contralateral to the preferred paw (dominant hand) to prevent usage of the non-dominant paw. Rats were then trained to perform trials consisting of approaching the slit, reaching for the pellet, eating the pellet, and turning and walking to the rear of the chamber. Turns were encouraged by dropping a pellet in the rear of the chamber after the reach as needed. Redundant reaching (i.e., reaching without a pellet present) was discouraged by having the rat approach an empty shelf and not placing a pellet on the shelf until he performed a complete trial (approach and turn) without reaching [12].
Testing-staircase
Rats were placed in the Montoya Staircase Box, each test lasting 5 minutes. A single chocolate chip was placed on each of the 8 steps per side. The number of chocolate chips retrieved was counted. The test was repeated 3x unless the rat successfully retrieved all 8 chocolate chips on each side; the most successful trial for both the dominant and non-dominant hands was recorded and used to calculate skilled reaching ability. Data are presented as the mean number of treats successfully retrieved.
Testing-skilled forelimb reaching
Rats were placed in the single pellet reaching task box. A “warm-up” period of 10 trials was performed, followed immediately by the “testing” period of 20 trials. A trial was considered a success if the rat grasped the pellet and brought it into the box and ate it without dropping it. A trial was considered a failure if the rat failed to grasp the pellet (i.e., he knocked the pellet out of reach) or, after grasping the pellet, he dropped the pellet before eating it. We recorded the number of successful trials and the number of reaches a rat performed. We calculated the percent of successful trials ([number of successful trials/20]x100), the accuracy of each rat ([number of successful trials/number of non-redundant reaches during the session]x100), and the percentage of “first-reach” successful trials ([number of trials in which the pellet was successfully retrieved in a single reach/20]x100) [12].
Stroke surgery
Rats were initially anesthetized with 5% isoflurane in oxygen and then maintained with 1-3% isoflurane. Body temperature was maintained at 37°C with a heating pad controlled by a feedback system attached to a rectal probe. Bupivacaine, 3mg/kg, was given subcutaneously at the site of the incision. A small craniotomy was made 2mm posterior to Bregma, at 3mm to the right or left of the midline depending on the handedness preference displayed by the rat (i.e., right-handed rats had craniotomies performed to the left of the midline). Endothelin-1, 0.0625ug/uL, was injected 6mm beneath the skull at a 10 degree angle towards the coronal suture using a 26G needle attached to a Hamilton syringe (Hamilton). This placement targeted the ventral posterolateral and ventral posteromedial nuclei of the thalamus. The needle remained in place for 3min before and after the injection. 7 rats received 2uL ET-1, 7 rats received 4uL ET-1, while 36 rats received 8uL ET-1 (8 rats for part 1 of the study, 28 rats for part 2). The rates of injection were 0.5uL/30sec, 1uL/30sec, and 2uL/30sec, respectively, for the 2uL, 4uL, and 8uL injections. The incision was closed and rats were given 0.02mg/mL buprenorphine subcutaneously and kept warm during recovery.
Perfusion, sectioning
Seven days post-stroke, the 19 out of 22 rats remaining in part 1 were anesthetized with 5% isoflurane in oxygen and perfused with saline followed by 4% paraformaldehyde (PFA, Sigma-Aldrich), and the brains were removed and allowed to fix in 4% PFA for 4 hours before being rinsed with phosphate-buffered saline (PBS, Fisher) and stored in 30% sucrose (Sigma-Aldrich) at 4°C until sectioning. The brain was frozen on dry ice and, starting at the genu of the corpus callosum, 50um coronal sections were made with a sliding microtome (Leica SM200 R). Sections were stored in a cryoprotective solution of 30% sucrose and 30% ethylene glycol (Fisher) at -20°C.
Histology-CV staining and Measure infarct volume
Sections were mounted on gelatin-coated slides and cresyl violet (Sigma-Aldrich) staining was performed on 16 serial sections, 600um apart. Slides were scanned on a flat-bed CanoScan LIDE 100 scanner at 600dpi (Cannon). The areas of the uninjured tissue of both hemispheres were measured using the polygon selection tool of ImageJ (NIH). The stroke volume was calculated by subtracting the volume of the un-infarcted ipsilesional hemisphere from that of the contralesional hemisphere. The percentage of tissue lost was calculated by dividing stroke volume by the volume of the contralesional hemisphere.
Statistics
Statistics were performed using GraphPad Prism version 6.03 for Windows (GraphPad Software, La Jolla, California, USA). Groups were compared using paired two-tailed Student’s t-test. A p-value of less than 0.05 was considered significant.
Results
Different volumes of ET-1 yielded infarcts of different volumes
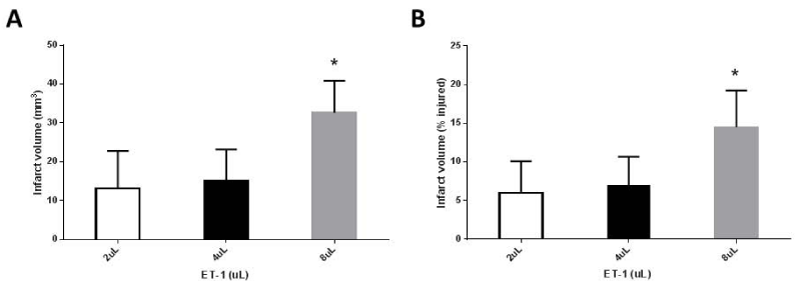
As can be seen in figure 2, injecting 2 or 4uL ET-1 creates a smaller infarct volume than injecting 8uL ET-1. The mean infarct volume for a 2uL ET-1 injection was 13.1 mm3, the mean infarct volume for a 4uL ET-1 injection was 15.1 mm3, and the mean infarct volume for an 8uL injection was 32.6 mm3. Although there was no significant difference between the mean infarct volumes of rats receiving 2 and 4uL injections (p=0.7203), the infarct volumes for both the 2uL and 4uL groups were significantly different from that of the 8uL group (p-values for 2 vs 8uL ET-1 and 4 vs 8uL ET-1 were p=0.0150 and p=0.0102, respectively). Similarly, the percentage of injured tissue relative to uninjured tissue was 6.0% and 7.0% for the 2 and 4uL groups versus 14.5% for the group of rats injected with 8uL ET-1 (p=0.0232 and p=0.0221, respectively).
Staircase test
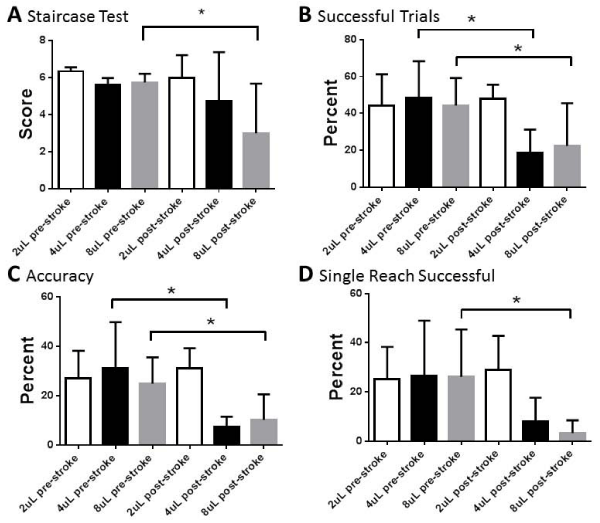
The staircase test was able to discern differences between pre- and post-stroke behavior for rats who received the 8uL ET-1 injection, but not for rats receiving the 2 or 4uL injection (Figure 3a). The mean pre-stroke score for rats receiving the 2uL injection was 6.340, while the mean post-stroke score was 6 (p=0.3723). The mean pre-stroke score for rats receiving the 4uL injection was 5.625, while the mean post-stroke score was 4.75 (p=0.2393). The mean pre-stroke score for rats receiving the 8uL injection was 5.75, while the mean post-stroke score was 3 (p<0.0001).
Skilled forelimb reaching test
2 out of 3 metrics show the reaching test is capable of distinguishing pre- and post-stroke differences in both 4uL and 8uL groups (Figure 3b-d). The 3rd metric, first reach successful, which is the most stringent test, is able to discern differences for the 8uL group. None of the metrics were able to distinguish pre- and post-stroke differences in the 2uL group.
The percentage of successful trials measure shows differences between the pre- and post-stroke score for both the 4uL and the 8uL groups (Figure 3b). The mean pre-stroke score for rats receiving 2uL ET-1 was 44.1% while the mean post-stroke score was 48% (p=0.6192). The mean pre-stroke score for rats receiving 4uL ET-1 was 48.41% while the mean post-stroke score was 18.75% (p=0.0056). The mean pre-stroke score for rats receiving 8uL ET-1 was 44.33%, while the mean post-stroke score was 22.5% (p=0.0018).
The percent accuracy was also able to discern pre- and post-stroke differences for these groups (Figure 3c). The mean pre-stroke score for rats receiving 2uL ET-1 was 27.09% while the mean post-stroke score was 31.0% (p=0.4502). The mean pre-stroke score for rats receiving 4uL ET-1 was 30.97% while the mean post-stroke score was 7.45% (p=0.0179). The mean pre-stroke score for rats receiving 8uL ET-1 was 24.87%, while the mean post-stroke score was 10.15% (p=0.0019).
The percentage of first-reach successful measurement was able to distinguish differences for the 8uL group but not the 4uL group or 2uL group (Figure 3d). The mean pre-stroke score for rats receiving 2uL ET-1 was 25.3% while the mean post-stroke score was 29% (p=0.5506). The mean pre-stroke score for rats receiving 4uL ET-1 was 26.67% while the mean post-stroke score was 8% (p=0.1085). The mean pre-stroke score for rats receiving 8uL ET-1 was 26.17%, while the mean post-stroke score was 3.333% (p=0.0055).
Effect of handedness
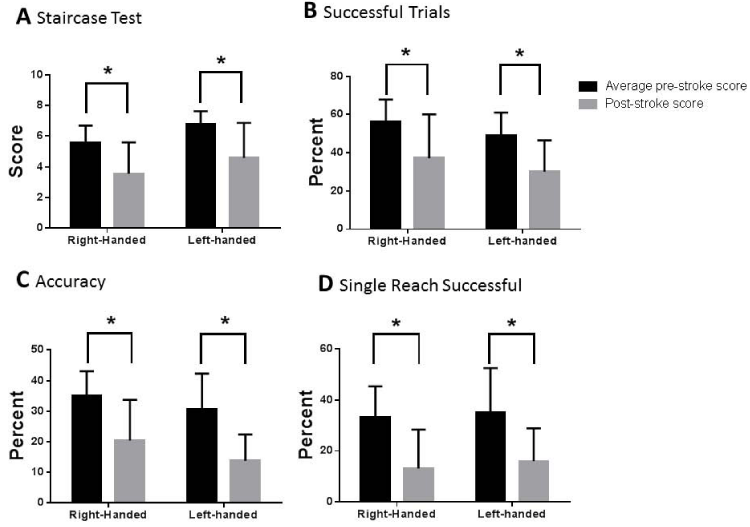
We allowed the rats to determine hand preference during training for the reaching test. This hand preference was then used to determine which hemisphere received the stroke (the contralateral hemisphere was injected with ET-1). We determined that while there were differences between how left and right handed rats injected with 8uL ET-1 performed on the tests, both tests were able to discern statistically significant differences between the pre- and post-stroke tests regardless of the rat’s hand preference (Figure 4).
Discussion and Conclusion
Fine motor skills of the hand are one of the most frequently lost motor functions following a stroke, making it difficult for patients to perform the most basic activities of daily living [6]. Even after extensive rehabilitation, many patients are not able to recover these behaviors [10]. Skilled reaching as a task is common to both humans and rodents although differences in the individual components of the movement do exist [14-16]. For example, rats use a “whole hand” approach while humans use a “pincher grasp” to grasp the target. Rats use olfactory cues to localize the target while humans use visual cues. Despite these differences between the species, it is thought that the neural pathways employed are comparable [14-16]. Thus, skilled reaching tasks have the potential to be valuable indicators of post-injury behavioral deficits and functional recovery.
Two of the most widely used skilled reaching tests are the staircase test and the skilled forelimb reaching test. While both tasks test the fine motor skills of the participant, they vary significantly when it comes to their cost in terms of money, time, and investigator involvement. The skilled forelimb reaching test box is a simple design which may be easily built by the experimenters or purchased. In contrast, the staircase test box is more complex and so it is likely to be purchased commercially.
Both tests require time for the animals to habituate to the box and a period of training, two weeks being the preferred amount of training time, but only the skilled forelimb reaching test requires extensive investigator involvement [18]. Because of the labor-intensive nature of the test, the skilled forelimb reaching test has a greater potential for rater-dependent variability than the staircase test. Some factors influencing the results of the test may be how quickly the pellets are placed between trials, how well pellets are placed, and variations in scoring, particularly if a kinematic analysis of the movement is performed. Therefore, the skilled forelimb reaching test requires not only training of the animal but also training of the investigator [18]. Thus, the staircase test, which can be completed and scored by an untrained observer, is considered less subject to operator bias [18].
The staircase test has another advantage in that both arms can be tested simultaneously, allowing the investigator to select which hemisphere in which to induce the stroke, and allowing a comparison of injured and uninjured behaviors in the same animal. Thus, each animal serves as its own control [18]. In contrast, in the skilled forelimb reaching test, the rat selects its preferred limb and is then trained exclusively on that side. Therefore, a single study must either use a larger number of animals, excluding those which prefer to use the ‘wrong’ hand, or it must include animals with injuries to either hemisphere.
Weaknesses of our study include a simplified system of measurement, the use of only one strain of rats, the use of two different treats as a reward, and lack of multiple post-injury time points. We chose to use the most simplified quantitative analysis of these tests; however, there are ways to increase the information gathered. For example, using multicolored pellets in the staircase test can provide not only the percent success rate but also indicate the distance which a rat is capable of reaching, a measure which cannot easily be made using identical pellets on each stair since the animals often displace the pellets during the test [21]. Additionally, both tests may be recorded to allow for a detailed examination of their movements. A qualitative kinematic analysis of each component of the reaching movement can provide insight into not only into which portion of the movement has been affected but also any compensatory behaviors the animal may utilize [18,19,22]. Nikkhah and Whishaw, among others, have both studied the effect that strain can have on the results of skilled reaching tests [23,24]. Nikkhah showed that there are significant differences among strains in the training, or ‘acquisition phase,’ of the staircase test [23]. Although Whishaw showed that there was no difference in terms of quantitative results, it was determined that Long-Evans and Sprague-Dawley rats employ different movements when executing their reach, which may have implications for the results of a qualitative analysis [24]. We used two different treats, mini-chocolate chips and sugar pellets, to increase the motivation of our animals. The average size of the mini-chocolate chips was 5/16 inch (0.79 cm) wide by 3/16 inch (0.48 cm) high; the average size of the sugar pellets was 3/16 inch (0.48 cm) wide by 2/16 inch (0.32 cm) high. It is possible that the larger size of the mini-chocolate chips made the task easier and thus masked any deficit that would have been seen in the animals which received the smaller volume injections. However, it should be noted that even with the larger treat, perfect scores on the staircase test were a rarity. Finally, the main weakness of our study is that we focused on only one time point post-injury. Due to the rapidly evolving immunological response, the prevalence of spontaneous recovery, and the need to study the therapeutic potential of different agents over the long-term, more time points would be beneficial [2].
The strengths of our study include the fact that we performed both tests in the same animals and that we used varied injection volumes. Although the repeated training and testing of both tasks in the same animals allows for a direct comparison of the tests it may also potentially increase the possibility of inadvertent rehabilitation. Anecdotally, it also showed us that while an animal may excel at one type of reaching test he may do poorly at another (data not shown). It is has been postulated that different sizes of injury, even if those injuries are of the same type, can affect the ability of a behavioral test to detect deficits in behavior [17,25]. Therefore, we tested the effects of increasing volumes of endothelin-1, hypothesizing that we would see a dose-response with regards to infarct volumes.
As can be seen from the results, the amount of damage incurred in the brain differed depending on the volume injected. An injection of 8uL of endothelin-1 leads to greater damage than an injection of 2 or 4uL. The skilled forelimb reaching test was able to detect differences in both the 4 and 8 uL groups, while the staircase test was only able to discern between pre- and post-injury rats in the 8uL group. These results suggest that the skilled forelimb reaching test is a more sensitive test than the staircase test and should potentially be the test of choice for those studying lacunar ischemic strokes.
The authors wish to thank Morgan Suhre, of the University of Wisconsin-Madison, for her contributions to the manuscript. This study was supported by a National Institutes of Health grant to Dr. Jensen (NINDS 1K08NS079622-01A1).
- Fluri F, Schuhmann MK, Kleinschnitz C (2015) Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther 9: 3445-3454. Link: https://goo.gl/1N0y7x
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, et al. (2009) Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40: 2244-2250. Link: https://goo.gl/JUsnim
- Jensen MB, Han DY, Sawaf AA, Krishnaney-Davison R (2011) Behavioral outcome measures used for human neural stem cell transplantation in rat stroke models. Neurol Int 3: e10. Link: https://goo.gl/AODZWn
- Alaverdashvili M, Whishaw IQ (2010) Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience 167: 21-30. Link: https://goo.gl/uZnppI
- Trueman RC, Diaz C, Farr TD, Harrison DJ, Fuller A, et al. (2016) Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: Tests for long-term assessment. J Cereb Blood Flow Metab 37: 1349-1361. Link: https://goo.gl/o5O5Yg
- Langhorne P, Coupar F, Pollock A (2009) Motor recovery after stroke: a systematic review. Lancet Neurol 8: 741-754. Link: https://goo.gl/DSRtaA
- Arboix A, Padilla I, Massons J, Garcia-Eroles L, Comes E, et al. (2001) Clinical study of 222 patients with pure motor stroke. J Neurol Neurosurg Psychiatry 71: 239-242. Link: https://goo.gl/WiAt5q
- Steinke W, Ley SC (2002) Lacunar stroke is the major cause of progressive motor deficits. Stroke 33: 1510-1516. Link: https://goo.gl/cV98Ij
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ (2003) Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 34: 2181-2186. Link: https://goo.gl/osJVM1
- Houwink A, Nijland RH, Geurts AC, Kwakkel G (2013) Functional recovery of the paretic upper limb after stroke: who regains hand capacity? Arch Phys Med Rehabil 94: 839-844. Link: https://goo.gl/5Hfgph
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB (1991) The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods 36: 219-228. Link: https://goo.gl/D5cbL9
- Whishaw IQ, Whishaw P, Gorny B (2008) The structure of skilled forelimb reaching in the rat: a movement rating scale. J Vis Exp. Link: https://goo.gl/cxRYc8
- Whishaw IQ, Pellis SM (1990) The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res 41: 49-59. Link: https://goo.gl/7jTJQC
- Klein A, Sacrey LA, Whishaw IQ, Dunnett SB (2012) The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neurosci Biobehav Rev 36: 1030-1042. Link: https://goo.gl/056ZoF
- Whishaw IQ, Pellis SM, Gorny BP (1992) Skilled reaching in rats and humans: evidence for parallel development or homology. Behav Brain Res 47: 59-70. Link: https://goo.gl/u9ONDn
- Sacrey LA, Alaverdashvili M, Whishaw IQ (2009) Similar hand shaping in reaching-for-food (skilled reaching) in rats and humans provides evidence of homology in release, collection, and manipulation movements. Behav Brain Res 204: 153-161. Link: https://goo.gl/XkKOjD
- MacLellan CL, Gyawali S, Colbourne F (2006) Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behav Brain Res 175: 82-89. Link: https://goo.gl/9Et3WK
- Klein A, Dunnett SB (2012) Analysis of skilled forelimb movement in rats: the single pellet reaching test and staircase test. Curr Protoc Neurosci Chapter 8: Unit8.28. Link: https://goo.gl/sB5QNj
- Clarke J, Ploughman M, Corbett D (2007) A qualitative and quantitative analysis of skilled forelimb reaching impairment following intracerebral hemorrhage in rats. Brain Res 1145: 204-212. Link: https://goo.gl/EzAWGQ
- Bailey EL, McCulloch J, Sudlow C, Wardlaw JM (2009) Potential animal models of lacunar stroke: a systematic review. Stroke 40: e451-458. Link: https://goo.gl/cz2dhg
- Kloth V, Klein A, Loettrich D, Nikkhah G (2006) Colour-coded pellets increase the sensitivity of the staircase test to differentiate skilled forelimb performances of control and 6-hydroxydopamine lesioned rats. Brain Res Bull 70: 68-80. Link: https://goo.gl/lD556j
- Alaverdashvili M, Whishaw IQ (2013) A behavioral method for identifying recovery and compensation: hand use in a preclinical stroke model using the single pellet reaching task. Neurosci Biobehav Rev 37: 950-967. Link: https://goo.gl/GgYSgV
- Nikkhah G, Rosenthal C, Hedrich HJ, Samii M (1998) Differences in acquisition and full performance in skilled forelimb use as measured by the 'staircase test' in five rat strains. Behav Brain Res 92: 85-95. Link: https://goo.gl/gDq6GX
- Whishaw IQ, Gorny B, Foroud A, Kleim JA (2003) Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav Brain Res 145: 221-232. Link: https://goo.gl/8rexHR
- Gonzalez CL, Kolb B (2003) A comparison of different models of stroke on behaviour and brain morphology. Eur J Neurosci 18: 1950-1962. Link: https://goo.gl/Juhlh1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
