Journal of Neurology, Neurological Science and Disorders
Exercise induced operant conditioning of the H-reflex in stroke patients: Hopes for improving motor function through inducing plastic changes in the spinal pathways
1Department of Kinesiology and Program in Neurosciences, Research Center for neuromodulation and pain, Indiana University Bloomington, USA
2Department of Kinesiology and Program in Neurosciences, Indiana University Bloomington, USA
Author and article information
Cite this as
Tahayori B, Koceja D (2019) Exercise induced operant conditioning of the H-reflex in stroke patients: Hopes for improving motor function through inducing plastic changes in the spinal pathways. J Neurol Neurol Sci Disord. 2019; 5(1): 001-005. Available from: 10.17352/jnnsd.0000026
Copyright License
© 2019 Tahayori B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Cerebrovascular accident is a major cause of disability. Stroke survivors suffer from various severity levels of movement impairment which would substantially affect their quality of life. Several methods have been investigated for improving movement in these patients. Most of the treatment approaches are geared toward inducing neuroplasticity in the brain. Here, we introduce a novel method to induce neuroplasticity in spinal cord to compensate the cerebral insult.
Purpose: The aim of this study was to examine the ability of hemiplegic stroke patients to volitionally down-regulate the soleus H-reflex and its functional consequence. A humancomputer interface was developed to monitor several neural and behavioral factors while subjects stood on a balance board. The interface would elicit an H-reflex when the criteria were met and would provide feedback to the patients about the amplitude of the H-reflex. Subjects were encouraged to down-regulate the amplitude of the reflex.
Results: The protocol was tested in 3 hemiplegic subjects. Subjects demonstrated the ability to down-regulate the H-reflex. The rate of success in this down-regulation was on average %80.1±9.96. This success rate was in strong agreement with improvement in gait symmetry and gait velocity.
Major findings: This study demonstrated that stroke survivors have the ability to down-regulate their spinal reflexes and this down-regulation was correlated with movement improvement. Conclusion. The results suggest that stroke patients have the ability to down-regulate the Hreflex amid corticospinal damages. This was accompanied by improvement in motor function.
Potential implications: The current study has provided proof of evidence to show that inducing plastic changes in the spinal cord can improve motor output in stroke survivors. This method could be another treatment approach for stroke impairment.
CVA: Cerebrovascular Accident; TA Tibialis Anterior; H-reflex: Hoffman Reflex; CPN: Common Peroneal Nerve; SL: Step Length; GII: Gait Improvement Index: SR: Success Rate
Introduction
A cerebrovascular accident (CVA) is a leading cause of death and disability worldwide [1]. In recent years, several modern rehabilitation methods have been introduced and successfully tested [2-7]. The significance of these attempts is that they have utilized new technologies to bring basic concepts in neuroscience into clinical trials. This is especially critical for patients who are assumed to have been plateaued or do not show substantial improvement with other traditional therapy methods.
In line with the recent endeavors in stroke rehabilitation, we designed a novel method with the purpose of inducing plastic changes in lower motoneurons to compensate the function of upper motoneurons. The potential of spinal circuits as a site for neurorehabilitation are largely ignored in stroke rehabilitation. Here we used a well-established notion from basic neuroscience (operant conditioning of reflexes) for clinical neurorehabilitation.
Operantly conditioning the stretch reflex or its electrical analogue, the H-reflex, can permanently down or up-regulate the amplitude of the reflex [8-10]. It is shown that patients with partial spinal cord injury are able to condition their reflexes [11]. The functional implications of this plastic change have also been investigated [12]; operant conditioning of the H-reflex improves the locomotion of rats with incomplete spinal cord injury [13]. This finding was also confirmed in human patients as well [14].
Method
Subjects
Three ischemic stroke survivors (83±13.12 y/o and 5.17±5.92 y/post stroke) participated in this study and completed the three week treatment protocol. Prior to participating in the study, all subjects provided written consent. This study was approved by the Institutional Review Board of Indiana University.
The study consisted of testing sessions and treatment sessions. The testing sessions were conducted at the beginning of the study and at the termination of training. In these sessions, electrophysiological and functional tests were administered to investigate possible changes in response to the treatment.
EMG and H-reflex data acquisition
For recording the electromyographic (EMG) activity of the soleus and tibialis anterior (TA) muscles, surface Ag-AgCl electrodes with the inter-disk interval of 20 mm were used. For soleus muscle recording, the electrode was placed on the lower portion of the muscle, closer to calcaneal tendon to avoid any cross talk from the gastrocnemii. Another recording electrode was placed over the motor point of the TA, in parallel to its muscle fibers. A reference electrode was place on the lateral malleolus of the affected side. Therapeutic Unlimited system was used to record the EMG signals.
The H-reflex was elicited using an S8800 GRASS stimulator (Natus Neurology Incorporated, Warwick, USA). A disposal electrode was placed above the patella and a ball electrode with a diameter of 20 mm was placed on the posterior aspect of the knee joint to stimulate the posterior tibial nerve.
The analog signals were digitized using a National Instruments AD board with the sampling rate of 4000 Hz. Customized programs were written in DASYLab environment (DASYTec USA a national Instrument Company, Norton, MA, USA) data capturing and online signal monitoring and analysis. Further data analyses were done in Matlab (MathWorks®, Natick, MA, USA) using custom-written codes.
Neurophysiological and functional testing
In the testing sessions the recruitment curve of the H-reflex was first obtained. By increasing the stimulation intensity, the peak to peak amplitude of the H-reflex increases first and then decreases due to the collision of nerve signals. However, the muscular response (The M-wave) increases as the intensity increases. From this curve, maximum H-reflex (H-max) and maximum muscular response (M-max) was determined. An H-reflex equal to about 30% of M-max was selected for operant conditioning.
To assess their locomotion, subjects walked on a Gait Trainer 3TM treadmill at their preferred speed for four minutes. This treadmill measured the Mean Walking Speed, Mean Step Cycle Time, Mean Step Length (SL) and the Coefficient of Variance (CV; the amount of variation occurring between footfalls).
A Gait Improvement Index (GII) was defined as follows:
Where CVaff and SLaff are the CV and SL of the affected side, respectively and CVun and SLun are the CV and SL of the unaffected side, respectively. The difference between the pre and post GII was calculated and used as a Relative Gait Improvement Index (RGII).
Human-computer interface
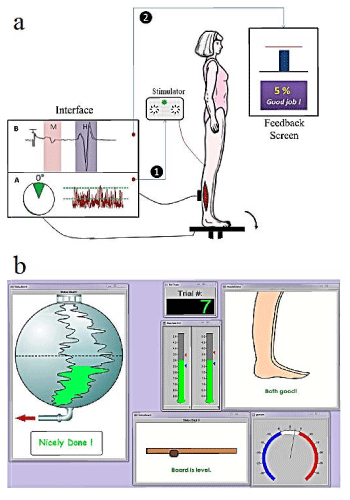
An interface was developed in DASYLab environment to conduct the protocol. This interface consisted of three steps: step one, to measure the baseline H-reflex value at the beginning of each treatment session. Step two, to measure the baseline EMG activity level. Step three, to perform the treatment protocol. A schematic diagram of the program is provided in figure 1. The subjects’ task was to balance the board without over-activating their soleus and TA muscle activity. At random intervals the posterior tibial nerve was stimulated to elicit and H-reflex and subsequent postural perturbation. Using biofeedback the subjects’ task was to reduce the perturbation by decreasing the amplitude of the H-reflex.
Training setup
In treatment sessions, subjects stood on a custom-designed balance board with an adjustable base. Subjects were initially trained to stand on the balance board and keep it balanced. To avoid falling during the exercise, a Biodex body support system was used to harness the subjects. Surface electrodes were placed bilaterally on the soleus and TA muscles to monitor the EMG activity of these muscles and to record the reflex response. An electrogoniometer was placed on the affected ankle joint to monitor the ankle joint angle.
The program would trigger the stimulator only when (a) the board was balanced, (b) the ankle joint was within 5 degrees from neutral in either direction, and (c) the EMG activity of the affected side was within 30% of the baseline values. The program also measured the amplitude of the H-reflex and provided a visual representation as feedback to the subjects on each trial. Subjects were encouraged to decrease the amplitude of the reflex. Whenever they were able to successfully down-regulate the reflex, the program would provide positive reinforcement. To avoid providing false positive feedback, the program would show them “success” whenever the H-reflex was depressed for more than 10% compared to the baseline value.
A separate monitor was placed in front of the subjects to provide them with feedback about their muscle activity, balance board status and the amplitude of the H-reflex after each trial. For each training session a Success Rate (SR) was calculated which is the percentage of successful trials over the total number of trials. SR was the ability of each subject to suppress this reflex when training.
Treatment schedule
Subjects practiced this exercise for three sessions per week for three consecutive weeks. In each treatment session subjects exercised in three blocks. Each block consisted of 100 H-reflex trials.
Results
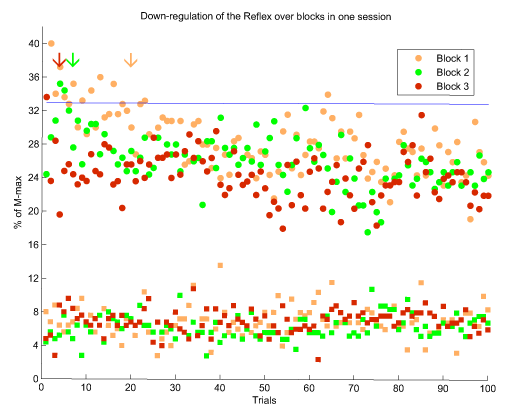
In each session, subjects practiced in three consecutive blocks. As the sessions progressed, they were able to better control their reflex amplitude. Figure 1 shows the three blocks of one of the final sessions of subject DH. As can be seen, the amplitude of the reflex was substantially depressed in all three blocks. However, as the blocks proceeded, successful down-regulation of the reflex occurred at earlier trials (as is indicated by arrows in fig 1, which are shifted to the left). The consistency of the M-wave ensures that the modulation of the H-reflex was not influenced by a biased input to the spinal circuits.
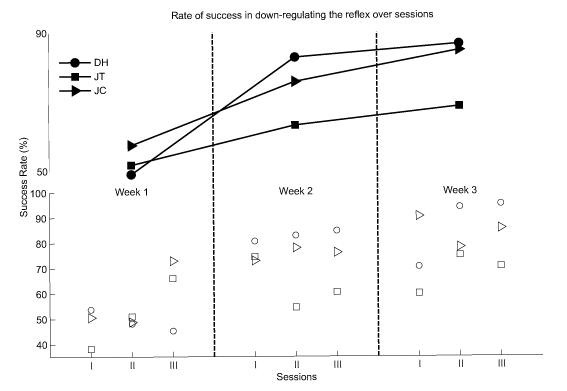
The rate of success of the subjects was calculated for each session and for each week. The success rates of DH, JT and JC at their first week of training were 49.00, 51.69 and 57.40, respectively. These values were 86.67, 68.58 and 84.85, respectively for the last week of their training. The success rate for all sessions and all weeks are presented in figure 2.
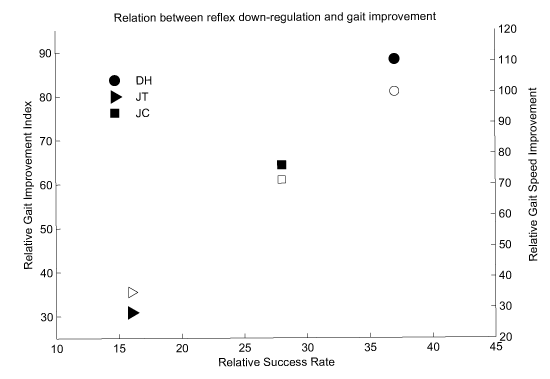
These results of gait evaluation are summarized in table 1. To better clarify that these improvements were related to the reflex conditioning, the Relative Success Rate (RSR) and Relative Gait Improvement Index (RGII) were calculated by subtracting SR and GII of the posttreatment from those of the pre-treatment, respectively. These are presented in figure 3. It can be seen that the improvement in function had a strong agreement with the success in downregulating the H-reflex (filled symbols). This was also the case between walking velocity improvement and the success in down-regulation (open symbols in figure 4).
Discussion
We used a basic neuroscience concept to prescribe an exercise protocol with the aid of programming technology. The framework of this idea was to introduce a novel therapy to regain voluntary control of motoneurons after a stroke.
The central goal of this study was to answer (1) whether patients with pyramidal tract disease possess the ability to down-regulate their reflexes and (2) whether inducing such a plasticity at the level of the final common pathway can influence the motor behavior profile of the subjects. Three adults with ischemic cerebrovascular accident participated in the study. These patients were able to down-regulate the amplitude of their H-reflex with the success rate improving throughout the course of the exercise. It is known that severing the corticospinal tract in animal models prevents them from being able to down-regulate the stretch or the H-reflex. In stroke patients, although the insult took place within the pyramidal system, these patients were shown to be able to modulate their reflexes. However, based on the results of this study, it cannot be inferred whether all types of stroke patients have this ability. The site and extent of the insult could be a determining factor for down-regulating the reflexes; an important question to be answered.
In a stroke, the inhibitory control that the cortex exerts over the spinal cord is decreased which results in an imbalance between the descending and the sensory inputs to the alpha motoneurons. Due to the loss of several inhibitory mechanisms in the spinal cord, the patient is not able to appropriately activate the alpha motoneurons and therefore, cannot properly perform deliberate and intentional motor tasks. Inducing a positive plastic change in these pathways seems to be promising avenue for rehabilitation. Here, we showed that inducing this plastic change is possible in stroke survivors. More importantly, this plastic change was accompanied by improvement in motor performance as was observed in their walking speed and RGII. Patients reported “feeling better in daily activities” after the second week of training. This observation is crucially important and impactful for researchers who work on rehabilitation methods for stroke survivors.
The duration of treatment in our protocol falls within the first phase of plastic changes associated with reflex down-regulation [15,16]. During this phase, plastic changes are reversible and do not cause a long lasting depression in the amplitude of the reflex. Nonetheless, functional improvements were observed in this first phase. Further studies with larger sample sizes and longer durations are warranted to investigate the possibility of permanent changes in the reflex pathways and their functional consequences.
This study did not directly investigate the mechanisms involved in the improvement of function. We only have some preliminary data for this part to propose a possible mechanism. Sensory inflow can also be regulated through the complicated mechanism of presynaptic inhibition of Ia afferents which acts to regulate the amount of neurotransmitter release [17]. It is also known that presynaptic inhibition has a critical role in motor control [18-20]. Surprisingly, it has been repeatedly demonstrated that this mechanism is not substantially affected in stroke patents [20-25]. Therefore, an increase in presynaptic control of Ia afferent could prevent the interruption of cortical drive and hence encourage volitional motor commands.
Conclusion
We combined computer technology and basic neuroscience knowledge to provide a novel treatment method for clinical rehabilitation of stroke survivors. Our data strongly suggest the ability of these patients to down-regulate the amplitude of the H-reflex and induce a plastic change at the level of alpha motoneurons. This was accompanied by improvement in gait. This method, in all likelihood, provides better volitional control over the lower motoneurons without changing their excitability level and/or spasticity level.
While our study provided data to answer the two main questions that we had, it opened many other questions that should be investigated for this potentially strong method for clinical rehabilitation of stroke.
Financial disclosure
This research was supported by an FRG-23 ACSM Foundation Research Grant from the American College of Sports Medicine Foundation. The first author received a Pre-Doctoral Fellowship from the American Heart Association (13PRE14780094). This work was also supported by Indian State Department of Health.
Funding
This research was supported by an ACSM Foundation Research Grant from the American College of Sports Medicine Foundation. The first author received a Pre-Doctoral Fellowship from the American Heart Association. This work was also supported by Indian State Department of Health.
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. (2012) Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation 125: 188-197. Link: https://goo.gl/67vEZq
- Tanaka N, Saitou H, Takao T, Iizuka N, Okuno J, et al. (2012) Effects of gait rehabilitation with a footpad-type locomotion interface in patients with chronic poststroke hemiparesis: a pilot study. Clinical Rehabilitation 26: 686-695. Link: https://goo.gl/rqsxXh
- Joo LY, Yin TS, Xu D, Thia E, Chia PF, et al. (2010) A feasibility study using interactive commercial off-the-shelf computer gaming in upper limb rehabilitation in patients after stroke. Journal of Rehabilitation Medicine 42: 437-441. Link: https://goo.gl/VvUjnq
- Lange B, Flynn S, Proffitt R, Chang C-Y, Rizzo AS (2010) Development of an interactive game-based rehabilitation tool for dynamic balance training. Topics in Stroke Rehabilitation 17: 345-352. Link: https://goo.gl/ZShtw3
- Volpe B, Krebs H, Hogan N, Edelstein L, Diels C, et al. (2000) A novel approach to stroke rehabilitation Robot-aided sensorimotor stimulation. Neurology 54: 1938-1944. Link: https://goo.gl/hwhf11
- Fung J, Richards CL, Malouin F, McFadyen BJ, Lamontagne A (2006) A treadmill and motion coupled virtual reality system for gait training post-stroke. CyberPsychology & behavior 9: 157-162. Link: https://goo.gl/8Erwvn
- Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE (1998) A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke 29: 1122-1128. Link: https://goo.gl/JvARFX
- Wolpaw JR (1987) Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol 57: 443-459. Link: https://goo.gl/PgP6Cb
- Chen XY, Wolpaw JR (1995) Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411-415. Link: https://goo.gl/pEDzBb
- Wolpaw J, Lee C, Calaitges J (1989) Operant conditioning of primate triceps surae Hreflex produces reflex asymmetry. Experimental Brain Research 75: 35-39. Link: https://goo.gl/y4UfU4
- Segal RL, Wolf SL (1994) Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol 130: 202-213. Link: https://goo.gl/ipwXr3
- Thompson AK, Wolpaw JR (2013) Operant Conditioning of Spinal Reflexes for Motor Rehabilitation after CNS Damage. Introduction to Neural Engineering for Motor Rehabilitation 549-570. Link: https://goo.gl/wQKcq1
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, et al. (2006) Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci. 26:12537-1243. Link: https://goo.gl/1xDaue
- Thompson AK, Pomerantz FR, Wolpaw JR (2013) Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365-2375. Link: https://goo.gl/Ruyn4G
- Wolpaw JR, O'Keefe JA (1984) Adaptive plasticity in the primate spinal stretch reflex: evidence for a two-phase process. J Neurosci 4: 2718-2724. Link: https://goo.gl/VLfu7c
- Wolpaw JR, Maniccia DM, Elia T (1994) Operant conditioning of primate H-reflex: phases of development. J Neurosci 170: 203-207. Link: https://goo.gl/Ygcz6c
- Tahayori B, Koceja DM (2012) Activity-Dependent Plasticity of Spinal Circuits in the Developing and Mature Spinal Cord. Neural plasticity 2012: 1-12. Link: https://goo.gl/qmmQc9
- Grillner S, Jessell TM (2009) Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19: 572-586. Link: https://goo.gl/8ven5r
- Tahayori B, Port NL, Koceja DM (2012) The inflow of sensory information for the control of standing is graded and bidirectional. Exp Brain Res 218: 111-118. Link: https://goo.gl/rkwZvN
- Pierrot-Deseilligny E, Burke DC (2005) The circuitry of the human spinal cord: its role in motor control and movement disorders. Cambridge Univ Press. Link: https://goo.gl/WgSxoH
- Burke D, Ashby P (1972) Are spinal “presynaptic” inhibitory mechanisms suppressed in spasticity? Journal of the neurological sciences 15: 321-326. Link: https://goo.gl/bXMD3A
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E (1994) A quantitative assessment of presynaptic inhibition of la afferents in spastics. Brain 117:1449-1455. Link: https://goo.gl/kCQxVu
- Katz R (1999) Presynaptic inhibition in humans: a comparison between normal and spastic patients. J Physiol Paris 93: 379-385. Link: https://goo.gl/Po2mkh
- Aymard C, Katz R, Lafitte C, Lo E, Pénicaud A, et al. (2000) Presynaptic inhibition and homosynaptic depression. Brain 123: 1688-1702. Link: https://goo.gl/ns9dqa
- Lamy JC, Wargon I, Mazevet D, Ghanim Z, Pradat-Diehl P, et al. (2009) Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain 132: 734-748. Link: https://goo.gl/cYMNCF
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
