Journal of Neurology, Neurological Science and Disorders
Suspicion of a teenager with Duchenne disease in the pediatrics department of Sainte Thérèse De Hinche Hospital
Berley Alphonse1*, Michelande Elien1, Edisond Florial2, Andorie Samanda Labonte3, Chrismy Augustin2 and Phélès Dagerus4
2University Notre Dame d’Haiti, Haiti
3University LUMIÈRE, Haiti
4Escuela Latino Americana de Medicina CUBA
Cite this as
Alphonse B, Elien M, Florial E, Labonte AS, Augustin C, et al.(2023) Suspicion of a teenager with Duchenne disease in the pediatrics department of Sainte Thérèse De Hinche Hospital. J Neurol Neurol Sci Disord 9(1): 041-044. DOI: 10.17352/jnnsd.000055Copyright
© 2023 Alphonse B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Duchenne disease is caused by a deletion of the gene coding for dystrophin. The absence of this protein is responsible for the myonecrosis observed during the evolution of the pathology. It is X-linked recessive which explains its occurrence, especially in boys. The manifestation of the disease begins in 3 years - 5 years, and the life expectancy is 20 years - 30 years.
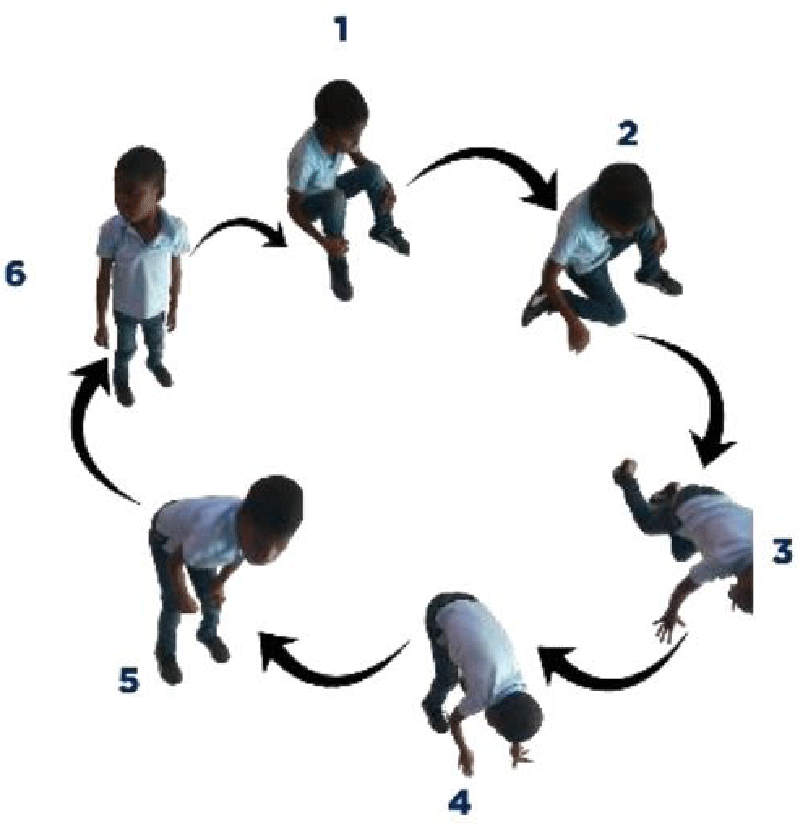
In the classic clinic, we have gait disorders, kyphoscoliosis, pseudo hypertrophy of the calves, and a positive Gower’s sign. Cardiorespiratory impairment is often incriminated as the cause of death in these patients. Management is based on physiotherapy and corticosteroid therapy. In the event of a cardio-respiratory manifestation, the use of positive pressure ventilation, and anti-hypertensives such as ACE inhibitors and beta-blockers may be necessary.
We will discuss the 10 years old patients who present with the classic symptoms of Duchenne disease and how we managed it in a low-income country.
Introduction
Duchenne muscular dystrophy (DMD) is a genetic disease caused by a “frameshift” deletion or a nonsense mutation in the DMD1 gene found on the X chromosome coding for dystrophin [1-8].
From an epidemiological point of view, this affects 1/3500 - 5000 newborns with a male predominance [9-11].
The Duchenne disease is a rare disease worldwide. It may go unnoticed by many clinicians. Thus, we will present the case of this 10-year-old boy with DMD. Hence, the objective is to discuss the method of diagnosis of Duchenne muscular dystrophy and its management.
Presentation
Ten-year-old patient, seen under referral from the Thomonde Health Center for mental retardation and functional limitation in walking. He was assessed by the pediatric team of the Sainte Thérèse de Hinche Hospital (HSTH)3 with the following reasons for consultation: language delay, functional limitation in walking, difficulty in standing up, the notion of repeated falls, abdominal pain, deformation of the spine and lower limbs. He had some learning difficulties.
The symptomatology would have started with the appearance of a language delay, with the notion of repeated falls, difficulty in standing up and walking around, and non-specific abdominal pain, neither tenesmus nor strains. As a history, he was hospitalized and treated for a neonatal infection and a seizure at the age of 2 years.
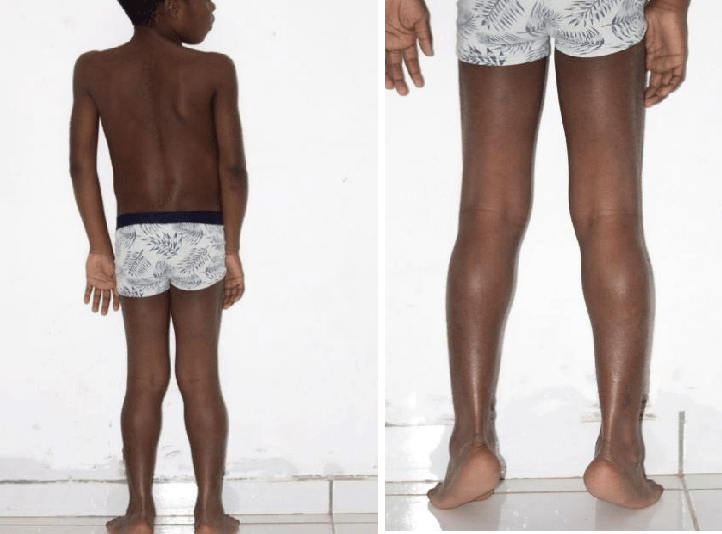
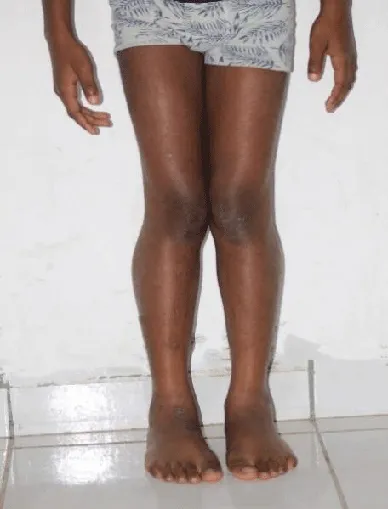
On physical examination, the patient had a weight of 24 kg and presented a deviation of the spine and an inequality of the left lower limbs: 64 cm and right: 65 cm. He couldn’t take support on his heel. He presented with lower limb paresis and Genu valgum. Gower’s sign was positive with pseudo-calf hypertrophy.
Cranial nerve assessment was unremarkable and muscle tone was normal.
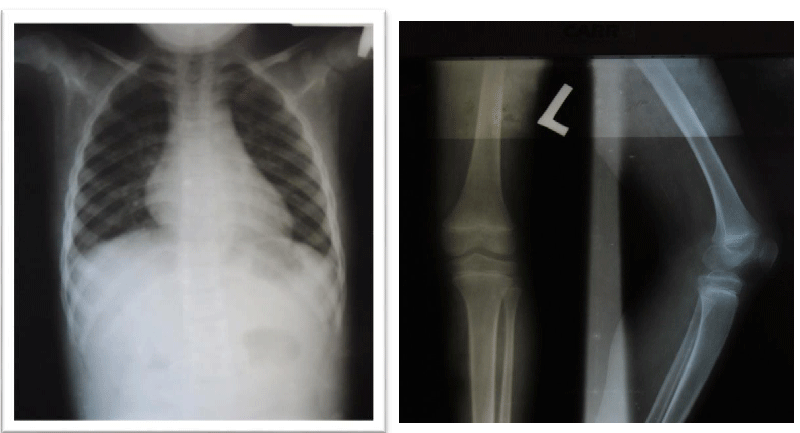
The patient underwent a radiological evaluation and biological investigations. A spine x-ray revealed lumbar scoliosis. The serological analysis itself revealed an increase in the CK-MB and Aldolase markers. Electromyography, biopsy, and genetic analysis could not be performed. The counseling was carried out with the patient’s mother and then he was referred to physiotherapy after an initiation to corticosteroid therapy. The patient was assessed several times during the follow-ups, and he has some degree of clinical improvement. However, there is worsening muscle weakness in the lower limbs. As the goal of corticosteroid therapy is to delay complications, we decided to continue with the same treatment as we do not have other alternatives.
Discussion
Dystrophin is the largest coding protein in the human genome [12]. It allows the anchoring and support of muscle fibers via their binding between the intracellular cytoskeleton (actin) and transmembrane alpha and β-dystroglycan proteins as well as their connection with the extracellular matrix [13-15].
The absence of dystrophin would cause progressive damage to myofibers with each muscle contraction [16]. This would explain the appearance of the clinical signs of the disease only from the age of 5 years and the variation of the clinical picture of the disease [17]. The repair of damaged muscle tissue will lead to the formation of fibrosis which can be observed on biopsy [18].
At first, the child presents with gait disturbances, delayed psychomotor development, and poor head control.
The study carried out by Kara Mirski, et al. on 179 boys diagnosed with Duchenne’s disease that the delay in walking could help in the early diagnosis of the disease associated with cognitive impairment.
With the evolution of the disease, there will be neuromuscular and bone manifestations as master symptoms of muscle weakness, pseudo hypertrophy of the calves, and deformation of the spine and other cardiovascular, respiratory, digestive, endocrine, urinary, neurological, and psychiatric [19].
In our case, the patient presented with a delay in psychomotor development, difficulty in walking, repeated falls, deviation of the spine, a positive Gower’s sign, inequality and paresis of the lower limbs, and pseudo hypertrophy of the calves.
We could not make a definitive diagnosis because we have some laboratory issues such as biopsy, electromyography, and genetic analysis. The suspicion was high because of the clinical presentation and the plasma elevation of CK-MB and Aldolase caused by the increased permeability of the damaged sarcomeres, due to muscle contractions thus causing their release into the circulation.
The standard management of DMD consists of physiotherapy and corticosteroid therapy which could delay the progression of the disease [5,20].
As perspectives, scientists are working on other therapeutic modalities such as exon skipping, gene therapy and myostatin inhibitors. In our context the management was focused on physiotherapy, corticosteroid therapy and strict monitoring of side effects of corticosteroids. We encourage the development of partnerships in the field of pediatric neurology in order to find a solution to this disease.
Conclusion
Duchenne disease is a rare genetic disease whose management is complex and aims above all to delay the progression of the pathology. We recommend all physicians who receive a boy with difficulty walking to measure CK-MB in order to detect the disease early.
Declaration of patient consent
The author certifies having had the authorization of the patient’s mother and the head of the HSTH pediatrics department, Dr. Dagerus Phélès.
Results section
- Gonçalves A, Fortuna A, Ariyurek Y, Oliveira ME, Nadais G, Pinheiro J, den Dunnen JT, Sousa M, Oliveira J, Santos R. Integrating Whole-Genome Sequencing in Clinical Genetics: A Novel Disruptive Structural Rearrangement Identified in the Dystrophin Gene (DMD). Int J Mol Sci. 2021 Dec 22;23(1):59. doi: 10.3390/ijms23010059. PMID: 35008485; PMCID: PMC8744749.
- Bencze M, Hou C, Periou B, Agbulut O, Gervais M, Ternacle J, et al. Receptor interacting protein kinase-3 promotes both myopathy and cardiomyopathy in dystrophin-deficient mice. bioRxiv. 2022. https://www.biorxiv.org/content/10.1101/2022.01.06.475271v1
- Duzhar VM, Radchenko VV, Gozhenko AI, Badiuk NS. Duchenne Muscular Dystrophy: Treatment with Fetal Progenitor Cell Transplant. Arch. 2021; 3:1225‑30.
- Kim A, Park M, Shin HI. Pain characteristics among individuals with Duchenne muscular dystrophy according to their clinical stage. BMC Musculoskelet Disord. 2022 Jun 4;23(1):536. doi: 10.1186/s12891-022-05504-5. PMID: 35659210; PMCID: PMC9166361.
- Butterfield RJ, Kirkov S, Conway KM, Johnson N, Matthews D, Phan H, Cai B, Paramsothy P, Thomas S, Feldkamp ML. Evaluation of effects of continued corticosteroid treatment on cardiac and pulmonary function in non-ambulatory males with Duchenne muscular dystrophy from MD STARnet. Muscle Nerve. 2022 Jul;66(1):15-23. doi: 10.1002/mus.27490. Epub 2022 Jan 20. PMID: 34994466; PMCID: PMC9197945.
- Villa C, Auerbach SR, Bansal N, Birnbaum BF, Conway J, Esteso P, Gambetta K, Hall EK, Kaufman BD, Kirmani S, Lal AK, Martinez HR, Nandi D, O'Connor MJ, Parent JJ, Raucci FJ, Shih R, Shugh S, Soslow JH, Tunuguntla H, Wittlieb-Weber CA, Kinnett K, Cripe L. Current Practices in Treating Cardiomyopathy and Heart Failure in Duchenne Muscular Dystrophy (DMD): Understanding Care Practices in Order to Optimize DMD Heart Failure Through ACTION. Pediatr Cardiol. 2022 Jun;43(5):977-985. doi: 10.1007/s00246-021-02807-7. Epub 2022 Jan 13. PMID: 35024902; PMCID: PMC8756173.
- Falzarano MS, Scotton C, Passarelli C, Ferlini A. Duchenne Muscular Dystrophy: From Diagnosis to Therapy. Molecules. 2015 Oct 7;20(10):18168-84. doi: 10.3390/molecules201018168. PMID: 26457695; PMCID: PMC6332113.
- Okubo M, Noguchi S, Hayashi S, Nakamura H, Komaki H, Matsuo M, Nishino I. Exon skipping induced by nonsense/frameshift mutations in DMD gene results in Becker muscular dystrophy. Hum Genet. 2020 Feb;139(2):247-255. doi: 10.1007/s00439-019-02107-4. Epub 2020 Jan 9. PMID: 31919629; PMCID: PMC6981323.
- Ortez C, Natera de Benito D, Carrera García L, Expósito J, Nolasco G, Nascimento A. Avances en el tratamiento de la distrofia de Duchenne [Advances in the treatment of Duchenne muscular dystrophy]. Medicina (B Aires). 2019;79 Suppl 3:77-81. Spanish. PMID: 31603849.
- Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M, Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017 Apr 26;12(1):79. doi: 10.1186/s13023-017-0631-3. PMID: 28446219; PMCID: PMC5405509.
- Erkut E, Yokota T. CRISPR Therapeutics for Duchenne Muscular Dystrophy. Int J Mol Sci. 2022 Feb 6;23(3):1832. doi: 10.3390/ijms23031832. PMID: 35163754; PMCID: PMC8836469.
- Lipscombe D, Pan JQ, Schorge S. Tracks through the genome to physiological events. Exp Physiol. 2015 Dec;100(12):1429-40. doi: 10.1113/EP085129. Epub 2015 Jul 19. PMID: 26053180; PMCID: PMC5008151.
- Lim KR, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther. 2017 Feb 28;11:533-545. doi: 10.2147/DDDT.S97635. PMID: 28280301; PMCID: PMC5338848.
- Jones L, Naidoo M, Machado LR, Anthony K. The Duchenne muscular dystrophy gene and cancer. Cell Oncol (Dordr). 2021 Feb;44(1):19-32. doi: 10.1007/s13402-020-00572-y. Epub 2020 Nov 14. PMID: 33188621; PMCID: PMC7906933.
- Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007 Jan;36(1):1-7. doi: 10.1016/j.pediatrneurol.2006.09.016. PMID: 17162189.
- Grounds MD, Terrill JR, Al-Mshhdani BA, Duong MN, Radley-Crabb HG, Arthur PG. Biomarkers for Duchenne muscular dystrophy: myonecrosis, inflammation and oxidative stress. Dis Model Mech. 2020 Mar 2;13(2):dmm043638. doi: 10.1242/dmm.043638. PMID: 32224496; PMCID: PMC7063669.
- Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM; DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018 Apr;17(4):347-361. doi: 10.1016/S1474-4422(18)30025-5. Epub 2018 Feb 3. PMID: 29395990; PMCID: PMC5889091.
- Bettica P, Petrini S, D'Oria V, D'Amico A, Catteruccia M, Pane M, Sivo S, Magri F, Brajkovic S, Messina S, Vita GL, Gatti B, Moggio M, Puri PL, Rocchetti M, De Nicolao G, Vita G, Comi GP, Bertini E, Mercuri E. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016 Oct;26(10):643-649. doi: 10.1016/j.nmd.2016.07.002. Epub 2016 Jul 11. PMID: 27566866.
- Emery AEH, Muntoni F, Quinlivan R. Duchenne Muscular Dystrophy. https://books.google.co.in/books/about/Duchenne_Muscular_Dystrophy.html?id=qAi3BgAAQBAJ&redir_esc=y
- Manzur AY, Kinali M, Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch Dis Child. 2008 Nov;93(11):986-90. doi: 10.1136/adc.2007.118141. Epub 2008 Jul 30. PMID: 18667451.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
