Open Journal of Parkinson's Disease and Treatment
Effectiveness of deep Brain Stimulation on early onset Dystonia: A Case Report
Ayse Unal, Ali Yalman, Filiz Altug* and Uğur Cavlak
Cite this as
Lalonde R, Strazielle C (2017) Effectiveness of deep Brain Stimulation on early onset Dystonia: A Case Report. Open J Parkinsons Dis Treatm 1(1): 032-038. DOI: 10.17352/ojpdt.000002Introduction
Dystonia is defined as a movement disorder characterized by involuntary muscular contractions that generate twisting and repetitive movements and/or abnormal postures [1]. The traditional clinical categorization of the dystonias based on age at onset, distribution of symptoms (focal, segmental, multifocal, generalized), and site of onset remains important and overlaps with the more recently employed genetic classification. Early-onset primary dystonia is a condition characterized by progressive problems with movement, typically beginning in childhood [2]. Early-onset dystonia often starts in a limb, tends to generalize and frequently has a genetic origin; whereas adult-onset dystonia usually spares the lower extremities, frequently involves cervical or cranial muscles with a tendency to remain focal, and appears sporadic in most cases [3].
Therapeutic strategies tend to vary with individual clinician preference and experience. As a general rule, in patients with generalized and multifocal diseases, oral pharmacotherapy constitutes the mainstay of treatment. Treatment options include benzodiazepines, clonidine, propofol, and deep sedation with barbiturates. Unfortunately the treatment of dystonia with oral agents is often unsatisfactory and injections of botulinum toxin are useful only in restricted areas (e.g., the face and neck) [4,5]. Surgical management, such as deep brain stimulation, will be required in up to one third of cases [6]. More recently, it has been used as a means of treating dystonic movement disorders. Bilateral pallidal DBS is the most popular target at this time for the treatment of dystonia. Deep-brain stimulation of the globus pallidus (GPİ-DBS) has been used successfully in primary generalized dystonia [7,8].
The aim of this study was to examine the effects of deep brain stimulation (DBS) surgery on severity of dystonia, hand function, and mobility in a patient with early-onset dystonia.
Case Report
This study carried out with a 11 years old female case for 1.5 years with complications of involuntary contractions. She applied to pediatric neurology department with walking disability and pain at left thigh and calf. When she was 10 years old, dystonia diagnosis has made. She received various medical treatments (Stalevo 50/12,5/200 mg 6 tablets per day, Lioresal 10 mg 3 tablets per day) for 1 year but the complications did not decrease. She had bilateral GPI-DBS operations when she was 11 years old.
Materials and Methods
Patient was assessed pre-operative and post-operative 10th day, 1st month, 3rd month and 6th month. Clinical assessments included Global Dystonia Rating Scale (GDS), Manual Abilty Classification System (MACS), Box and Block Test, Chair Stand Test, 10 Meter Walking Test.
GDS was used to assess the effects of patient’s dystonia on body parts and functions. The GDS rates dystonia severity in 14 body areas. The GDS is a Likert type scale with ratings from 0 to 10 (0 is no dystonia, 1 minimal, 5 moderate and 10 severe dystonia). The total score is the sum of the scores for all the body areas. The maximal total score of the GDS is 140 [9].
MACS levels are based on children’s ability to initiate handling objects by themselves (eating, dressing, playing, drawing or writing) and their need for assistance or adaptation to perform manual daily life activities that are appropriate for their age. The children at level I are able to handle objects easily, at level V, the children do not handle objects [10] and Box and Block Test were used to assess hand function[11]. Chair Stand Test and 10 Meter Walk Test were used to evaluate mobility.
Results
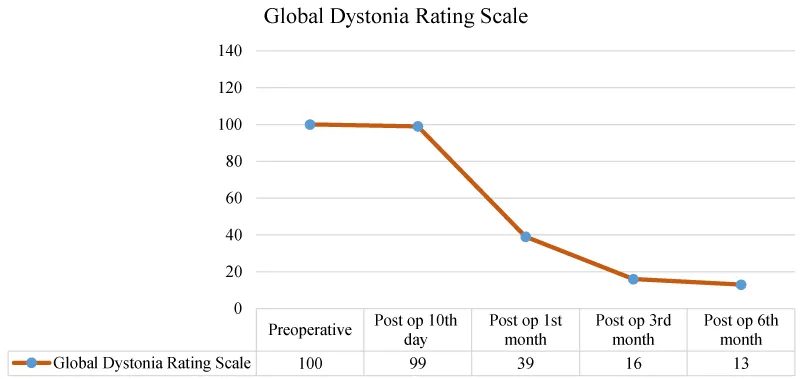
Pre-operative GDS score was 100. Post-operative GDS scores were 10th day 99, 1st month 39, 3rd month 16 and 6th month 13 (Figure 1).
Preoperative MACS score was 5. It was 5 for post-operative 10th day, 3 for post op 1st month, 3 for post op 3rd month and 2 for post op 6th month (Table 1).
Box and Blocks Test preoperative results were (box per minute) 4 for right hand, 2 for left hand. Post-operative Box and Blocks Test results were 10th day right 6, left 5; 1st month right 12, left 8; 3rd month right 14, left 9 and 6th month rigt 18, left 11 (Table 1).
Chair stand test and 10 meter walking test could not be performed on preoperative and post-operative 10th day. Chair stand test results (repeat per minute) were post-operative 1st month 4, 3rd month 5and 6th month 7 (Table 1).
10 meter walking test results (second) were post-operative 1st month 48.3 sec., 3rd month 45.2 sec, 6th month 42.1 sec (Table 1).
Discussion
Deep brain stimulation works most effectively for people who have an inherited form of dystonia or for idiopathic dystonia [12,13].
We discussed the effects of deep brain stimulation (DBS) on hand function and mobility in a patient with early-onset dystonia. All assessments were repeated preoperative, postoperative 10th day, 1st month, 3rd and 6th month. Preoperative GDS score was 100/140. The score gradually decreased in the postoperative period and it was 13/140 on postop 6th month. While she could not walk in the preoperative period, 10-Meter Walk Test results were 42.1 sec and chair stand test results were 7 repetitons, postoperative 6th month.
Groen et al. reported four patients with early onset dystonia who underwent bilateral GPi DBS. They observed 33% motor improvement on Burke-Fahn-Marsden Dystonia Rating Scale 6 months after surgery [14].
Kiss et al. performed a prospective, single-blind, multicenter study assessing the efficacy and safety of bilateral GPI-DBS in 10 patients with severe, chronic, medication-resistant cervical dystonia. Two blinded neurologists assessed patients before surgery and 12 months post-operatively using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS). The TWSTRS severity score improved at 12 months post-operatively [15,16].
The results of our study support and extend the results of previous studies, which also demonstrate that a patient with early-onset dystonia experiences robust responses to pallidal DBS with a 50% to 94% reduction in disease burden. Vidailhet et al. investigated the effects of bilateral pallidal stimulation on motor impairment, disability, quality of life, cognitive performance, and mood in a prospective multicentre 3 year follow-up study.
We found similarly results with literature. In a study performed by FitzGerald et al. patients were assessed using the Burke-Fahn-Marsden (BFM) Dystonia Rating Scale prior to surgery, 6 months after implantation and thereafter at 1 year, 2 years and 5 years follow-up. The group showed mean improvements in the BFM severity and disability scores of 43% and 27%, respectively, by 6 months, and this was sustained [17]. Seventy one patients with gait disorders, medically refractory dystonia underwent bilateral GPi DBS. Disability and mobility were assessed preoperatively and 6 months following surgery. They found that the disability score decreased, mobility level and hand functions improved [18].
Our results indicate that there is an improvement in the severity of dystonia, mobility, and measures of hand function after DBS, especially in the 1st month after surgery in a patient with dystonia. At six months after surgery; disability level decreased by 87%. Right hand function increased by %350, left hand function increased by %450 according to Box and Block test. Mobility scores (10 meter walking test) improved by %56.4.
We believe that further research in larger samples and long term follow-up results are needed to make about the effects of DBS surgery on the severity of dystonia, mobility, and hand functions in patient with early-onset dystonia.
Conclusion
DBS is a surgical procedure, which has positive effect on mobility and hand function in patient with early-onset dystonia. DBS for children should be offered early, preferably within a few years of the onset of symptoms, although the right timing will vary by individual.
- Tagliati M, Shils J, Sun C, Alterman R (2004) Deep brain stimulation for dystonia, Expert Review of Medical Devices 1: 33-41. Link: https://goo.gl/t70DOl
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, et al. (2008) The pathophysiological basis of dystonias. Nat Rev Neurosci 9: 222-234. Link: https://goo.gl/DCwCCd
- Klein C, Ozelius LJ (2002) Dystonia: clinical features, genetics, and treatment. Curr Opin Neurol 15: 491-497. Link: https://goo.gl/3rRA8y
- Bressman SB, Greene PE (2000) Dystonia. Curr Treat Options Neurol 2: 275–285. Link: https://goo.gl/peql88
- Vidailhet M, Vercueil L, Houeto JL, Houeto JL, Krystkowiak P, et al. (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467. Link: https://goo.gl/kn7Q8Z
- Fasano A, Ricciardi L, Bentivoglio AR, Canavese C, Zorzi G, et al. (2012) Status dystonicus: Predictors of outcome and progression patterns of underlying disease. Mov Disord 27: 783-788. Link: https://goo.gl/2dn4a6
- Lee JYK, Rezai A (2006) Deep brain stimulation of globus pallidus internus for dystonia: A review, Parkinsonism and Related Disorders 13: 261-265. Link: https://goo.gl/GAa2H3
- Lozano AM, Abosch A (2004) Pallidal stimulation for dystonia. Adv Neurol 94: 301-308. Link: https://goo.gl/HXu5fZ
- Comella CL, Leurgans S, Wuu J, Glenn ST, Chmura T (2003) Rating scales for dystonia: A multicenter assessment. Mov Disord 18: 303–312. Link: https://goo.gl/nWPlgk
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, et al. (2006) The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 48: 549-554. Link: https://goo.gl/aBjfT7
- Mathiowetz V, Federman S, Wiemer D (1985) Box and Block Test of Manual Dexterity: Norms for 6-19 Year Olds. Canadian Journal of Occupational Therapy 52: 241–245. Link: https://goo.gl/yr7N8g
- Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B (2000) Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. The Lancet 355: 2220–2221. Link: https://goo.gl/D9kOkL
- Kumar R, Dagher A, Hutchison WD, Lang AE, Lozano AM (1999) Globus pallidus deep brain stimulation for generalized dystonia: clinical and pet investigation. Neurology 53: 871–874. Link: https://goo.gl/bNQRpA
- Groen JL, Ritz K, Contarino MF, van de Warrenburg BP, Aramideh M, et al. (2010) DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord 25: 2420-2427. Link: https://goo.gl/TXCU5J
- Kiss ZHT, Doig-Beyaert K, Eliasziw M, Tsui J, Affenden A, et al. (2007) The Canadian Multicentre Study of Deep Brain Stimulation for Cervical Dystonia. Brain 130: 2879-2886. Link: https://goo.gl/UsI5tr
- Alterman RL, Tagliati M (2011) Deep Brain Stimulation for Dystonia: Patient Selection, Surgical Technique, and Programming. The Open Neurosurgery Journal 4: 29-35. Link: https://goo.gl/Dicu26
- FitzGerald JJ, Rosendal F, De Pennington N, Joint C, Forrow B, et al. (2014) Long-term outcome of deep brain stimulation in generalised dystonia: a series of 60 cases. J Neurol Neurosurg Psychiatry 85: 1371-1376. Link: https://goo.gl/3NZiRq
- Schrader C, Capelle HH, Kinfe TM, Blahak C, Bäzner H, et al. (2011) GPi-DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology 77: 483-488. Link: https://goo.gl/cAiAph
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
