Open Journal of Parkinson's Disease and Treatment
Accounting for behavioral deficits associated with damage in terms of cortical and subcortical information processes
Associate Professor, College of Engineering, Computing and Cybernetics, Australian National University, Canberra, ACT, Australia
Author and article information
Cite this as
Coward LA (2024) Accounting for behavioral deficits associated with damage in terms of cortical and subcortical information processes. Open J Parkinsons Dis Treatm . 2024; 7(1): 001-019. Available from: 10.17352/ojpdt.000014
Copyright License
© 2024 Coward LA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The way information about neurons in the brain is organized is critical to understanding how anatomical structures support cognition and why damage to specific anatomical structures results in specific deficits. Theoretical considerations indicate that the architecture of the brain has been constrained into some specific forms, and these forms make it possible to organize neuroscience information to achieve understanding. Different anatomical structures specialize in different information processes, where the information processes performed by one structure will support many different types of cognitive processes. However, all information processes in the brain are of two general types, condition definition/detection, and behavioral recommendation definition/integration. Motor and cognitive processes are carried out by combinations of processes of these two types. Cortical structures specialize in condition definition/detection processes and subcortical structures specialize in behavioral recommendation definition/integration processes. Local circuits within the cortex-hippocampus-thalamus-basal ganglia-cerebellum system perform different detailed information processes of these types. Brain damage to a specific structure results in loss of the information processes performed by that structure. The different deficits resulting from Parkinson’s disease, Huntingdon’s disease, Hemiballism, Tourette’s syndrome, damage to the hippocampal system, and strokes affecting the cortex, thalamus, or cerebellum can be understood in terms of the loss of specific information processes performed by different brain structures.
A large amount of experimental information has been collected on the anatomy and connectivity of the cortex, thalamus, basal ganglia, hippocampus, and cerebellum, and the behavioral deficits that result from damage to these structures. This paper describes a different way to organize and think about this information, in such a way that the relationship between behavioral deficits and the anatomy and connectivity can be understood.
There are some well-known patterns in the connectivity between the cortex, basal ganglia, thalamus, and cerebellum [1-3]. Cortical areas generally identified on the basis of neuron layering and chemical properties can equally well be identified on the basis of their patterns of connectivity with different subcortical regions [4]. Furthermore, the motor and cognitive deficits observed following damage to regions of the thalamus, basal ganglia and cerebellum that interconnect with a cortical area are very similar to the deficits observed following damage to the area itself [4]. It is also interesting that although the hippocampal system is extensively interconnected with the cortex [5], damage to this system leaves motor skills largely unaffected [6].
Understanding how these connectivity patterns implement the information processing required to support motor and cognitive behaviors has been less clear. However, theoretical system architectural considerations indicate a way to achieve such understanding. Practical pressures place architectural constraints on any system that detects information conditions in the available information and associates such condition detections with behaviors [7]. These practical pressures include the need to limit the physical information processing resources required to carry out behavioral features and the need to be able to change some features without undesirable side effects on other features. For a learning system, the pressures result in modules organized in a specific architectural form, with each such module organized into submodules, submodules into sub-sub modules, and so on, forming a modular hierarchy. Each module performs somewhat different information processes on different information and contributes to many cognitive processes. However, all information processes are one of two general types: condition definition/detection or behavioral recommendation definition/integration [7]. In the case of brains, natural selection generates practical pressures, and different anatomical structures correspond with the modules of this theoretical architecture [7,8].
The existence of the architecture and the two general types of information processes makes it possible to create hierarchies of description that enable understanding of cognitive phenomena in terms of brain anatomy and physiology [9]. A cognitive phenomenon can be described end to end in terms of the interactions between the highest-level modules. Such a description is approximate because it omits the interactions within these modules, but can be understood because this omission reduces the information density of the description. A more precise description can be achieved by mapping parts of the high-level description into descriptions in terms of the interactions between the submodules of a module. However, the information density is much higher, and only a part of the phenomenon can be understood at one time at this level of detail. Yet more accuracy can be achieved by mapping to even more detailed modules and so on. The linkages between descriptions of different detailed parts can only be understood at a higher level. However, the existence of the two general types of information process makes it possible to shift easily between the different levels, making an intuitively satisfying understanding possible.
Although there are no resemblances between computing systems and brains, this approach is analogous to the way in which the designs of complex electronic systems are understood [8,9]. Hierarchies of description make it possible to understand system features in terms of transistor operations, with mapping between different levels of description made possible by the use of just two general types of information process on every level, in the case of computers these two types are data read/write and instruction.
Applied to the brain, the hierarchies of description approach can lead to an understanding of the cognitive and motor deficits resulting from diseases or damage affecting local brain structures. Parkinson’s disease, Hemiballism, Huntingdon’s disease, and Tourette’s; and the deficits following physical damage to the cortex, thalamus, cerebellum, and hippocampus can be understood in terms of the loss of specific groups of information processes. In this paper, descriptions of these deficits will be developed at the higher levels, with some general indications of how to map to more accurate detailed levels. Coward [9] provides a more detailed description of how the mapping process works for one type of cognitive process.
Constraints on brain architecture
Computing systems with trillions of components like transistors are understood in the sense that they can be designed and modified. Although there are no resemblances between brains and computing systems, the way the computing system design information is organized to make understanding possible has some critical lessons for how neuroscience information can be organized to make understanding of cognitive processes possible [7-9]. Brains process information derived from the environment, the state of the body, and the internal state of the brain itself. This information processing determines and implements appropriate behaviors. Effectively, a brain detects conditions in that currently available information and associates those conditions with behaviors. A computing system also detects conditions in its currently available information and associates those conditions with behaviors. However, in a computing system, all the conditions, behaviors, and the associations between them are specified in advance by a designer. In a brain, most of the conditions, behaviors, and associations between them are defined heuristically. Many of the required brain behaviors are therefore to change conditions and the associations between conditions and behaviors.
Computing system designs need to limit the total information processing resources required and need to add or change system features without introducing undesirable side effects on other features. These needs generate constraints on the system architecture [7]. One important constraint is that the physical information processing resources tend to be organized into a modular hierarchy. To minimize the resources needed to carry out system features, information processes must be collected into groups. Groups are selected on the basis that the processes are similar, in the sense that they can be performed on the same physical resources. A set of physical resources called a module, is optimized to perform all the processes in the group very efficiently. Modules are separated into submodules in which the information processes are even more similar and so on. Information exchange between modules is minimized as far as possible. Note that in general, any one module will provide processes in support of many different features, and any one feature will require information processes performed by many different modules. Hence modules will not correspond with system features. A second important constraint is that information processes must all be one (or a combination) of just two general types: instructions and data read/writes. In computing systems, some high-level modules exist that specialize in just one of the information processing types: processor modules specialize in instructions and memory modules specialize in data read/writes. A computing system writes conditions (i.e. data) detected in its available information, reads conditions defined by a designer, and compares them. If they match, an instruction associated with the condition by a designer is executed.
Analogous practical pressures also exist for brains. If the brains of two different species need to learn similar behaviors, but the brain of one species requires fewer information processing resources (like neurons), that species will have a natural selection advantage. If the brain of one species can learn new behaviors without interference with previously learned behaviors, that species will have a natural selection advantage over a species in which new learning significantly disrupts earlier learning. These pressures generate constraints on brain architectures, analogous with but qualitatively different from the constraints on computer architectures [7,8]. As a result of these constraints, the physical resources of the brain tend to be organized in a modular hierarchy, with different major anatomical structures corresponding with high-level modules, substructures corresponding with submodules, and so on. Information exchange between modules is minimized as far as possible. There are just two general types of information processes, but in the brain, these types are behavioral recommendations and conditions defined/detected. A brain defines conditions in its available information and detects any occurrences of currently defined conditions. Each currently detected condition is associated with a range of recommendations in favor of different behaviors, each recommendation having an individually determined weight. The behaviors with the largest total weight across all currently detected conditions are implemented.
In the brain, some high-level anatomical modules exist that specialize mainly in just one of the information processing types: the basal ganglia specialize in behavioral recommendations and the cortex specializes in condition define/detections.
As in the case of computing systems, the organization of information processing resources into a modular hierarchy and the limitations of information processes to two general types make it possible to create hierarchies of description linking cognitive phenomena with, ultimately, neuron activity [9]. High-level descriptions of cognitive processes in terms of major anatomical modules are approximate but have a low enough information content that they can be fully understood. More precise descriptions can be created at the sub-module level, but the higher information content means that only part of the description of a cognitive process can be comprehended at this level. However, because of the use of just two general types of information processes, descriptions can be readily mapped between levels [9]. For example, the relationship between descriptions of different detailed parts can be understood at a higher level. This approach can be extended to even more detailed descriptions. Hence an intuitively satisfying understanding of cognitive processes in terms of anatomy and physiology can be achieved by shifting between the different levels of description as required.
High-level brain architecture
The high-level architecture resulting from the constraints is illustrated in Figure 1 [8]. There is a major separation between a module that defines and detects conditions in the available information, and a module that interprets each current condition detection as a range of recommendations in favor of different behaviors and implements the behaviors with the largest total recommendation weights. Some behaviors are externally directed like motor movements, but many behaviors act on the brain itself, releasing information between different brain structures or changing the connectivity between structures. There are five different general types of behavior. One type is the release of sensory information into the brain for processing (i.e. attention behaviors). A second type is the release of the results of processing by one anatomical structure for processing in another structure (i.e. cognitive processing behaviors). A third type is the release of the results of processing out of the brain to drive muscle movements (i.e. motor behaviors). A fourth type makes changes to some conditions (i.e. memory behaviors). A fifth type makes changes to the recommendation weights associated with recently detected conditions (i.e. reward behaviors). All these types must be sufficiently recommended by condition detections to be implemented.
As shown in Figure 1, the cortex is a module specializing in condition definition/detection processes and the subcortical structures form a module specializing in behaviors and recommendation definition/integration processes. Each of these modules has submodules that perform information processes supporting different aspects of these general information processes. In addition, there is a third major module called the cerebellum. This module specializes in processes that speed up the implementation of sequences of behaviors that have previously been learned between the two primary modules.
Condition definition/detection module: The cortex forms the condition definition/detection module and is made up of many separate areas, plus the cortex-like part of the amygdala. Each cortical area gets most of its inputs from a small number of other areas, plus in the case of the primary sensory areas from one of the senses. An area defines and detects conditions that are combinations of these inputs. Different cortical areas define and detect conditions in a different range of complexity, where complexity can be roughly understood as the total number of raw sensory inputs that contribute to the condition, directly or via conditions in intermediate areas. Although condition detection in any area could contribute recommendation strength to any behavior, conditions in a specific range of complexity are somewhat more effective for recommending specific types of behaviors.
Behaviors al recommendation definition/integration module: Subcortical structures form the behaviors al recommendation definition/integration module, including the basal ganglia, thalamus, basal forebrain, and hypothalamus. The striatum of the basal ganglia gets inputs from the cortex and interprets those inputs as behaviors and recommendations. The general type of behavior varies across the striatum from ventral to dorsal. The ventral striatum interprets its cortical inputs as recommendations in favor of more strategic behaviors, and recommendations become more specific when moving dorsally, with the most dorsal end interpreting inputs as recommendations in favor of specific motor movements. For example, a player in a team game like soccer must constantly select behaviors along this behavioral spectrum. Choosing to play defensively rather than attacking would be a strategic behavior selection. Choosing to move towards a specific area of the playing field would be a tactical selection. The detailed muscle movements to make a move would be detailed selections.
These behavior selections are implemented by the release of the cortical conditions that recommended the behaviors out of the cortical area in which they were detected. For example, condition detections in some cortical areas are most effective for recommending more strategic behaviors. The selection of one strategic behavior is implemented by the release of the conditions that recommended it to cortical areas effective for recommending more tactical recommendations. Conditions in these target areas will therefore tend to recommend a range of tactical behaviors of the already selected strategic type.
Once selected, releases are implemented by the thalamus. There are positive feedback loops between different cortical areas and different dorsal nuclei of the thalamus, but the basal ganglia constantly inhibits all these loops via the dorsal nuclei. When a behavior is selected by the basal ganglia, the inhibition of the corresponding dorsal nucleus is reduced, releasing activity in the corresponding cortical area to appropriate targets.
The amygdala and hypothalamus bias the cortex in favor of generating recommendations in favor of certain general types of behaviors, these biases are experienced as emotions. For example, anger is the subjective experience of the presence of strong recommendation strength in favor of aggressive behaviors. The cortex-like amygdala defines and detects conditions effective for recommending these different general types of behaviors. These conditions have recommendation strengths in both the striatum and in a different part of the amygdala that resembles the striatum. The striatal-like amygdala controls outputs from the hypothalamus to the cortex. Acceptance of such recommendations is implemented by the thalamus releasing activity in the cortex-like amygdala to other cortical areas and/or by the hypothalamus targeting different cortical areas.
Reward behaviors change the recommendation weights that result in recently accepted behaviors. Some cortical conditions recommend such reward behaviors, and if there is sufficient total recommendation weight the behaviors are implemented, either increasing or decreasing targeted recommendation weights.
Sometimes the exact timing of a release of cortical activity is important, and in these situations, the basal forebrain manages that timing. This is particularly the case for the behaviors that change the definitions of cortical conditions.
Condition change management module: In order to ensure a high integrity behaviors selection, at least a minimum number of recommendations must be available. In other words, at least a minimum number of cortical conditions must be detected. If too few conditions are being detected, the range of circumstances that define some undetected conditions must be expanded so that the conditions are detected. Because any one condition recommends a range of different behaviors, any such changes to the definition of that condition could jeopardize the integrity of all those recommendation strengths. These changes must therefore be carefully managed. Changes are recommended by conditions detected in the three areas associated with the hippocampus and selected by the hippocampus proper [10,11].
Behaviors sequence management module: The processing of cortical inputs through the basal ganglia to select a behavior takes a certain amount of time. Behaviors al responses can be speeded up for sequences of behaviors that often occur in the same order by shifting control to the cerebellum. Examples of such sequences are the sequences of muscle movements used for walking, climbing stairs, playing musical instruments, riding a bicycle, or speaking often-used words or phrases. Initially, these sequences are learned through the cortical conditions detected following one muscle movement acquiring recommendation strengths in the basal ganglia in favor of the next muscle movement. Once learned, the sequence can be shifted to the cerebellum. The sequence as a whole is then selected by the basal ganglia, and executed by the cerebellum, avoiding the time required for selection of each individual behavior by the basal ganglia. For each behavior, the cerebellum detects conditions defined by whatever combinations of cortical conditions happened to be active at the time the behavior was originally initiated by the basal ganglia. The cerebellum then speeds up execution by shifting these definitions so that they are detected somewhat earlier.
Connectivity within major brain modules
As a result of natural selection pressures in favor of efficient use of information processing resources, anatomical resources are organized in modular hierarchies [8]. Each major module specializes in a general type of information process. Submodules of the major modules specialize in subsets of the information processes performed by the major module. These subsets are groups of similar processes, where the similarity makes it possible to share the resources required to perform the processes. Sub-submodules of the submodules perform sub-subsets that are even more similar and so on.
Cortex
The major submodules of the cortex are Brodmann areas. Brodmann originally identified 44 areas, but more recent work identifies 150 – 200 such areas [12]. Areas are subdivided into columns [13].
Each column is made up of a sequence of about five layers of pyramidal neurons. These layers are numbered by Roman numerals II, III, IV, V, and VI. Inputs from the thalamus and from other cortical columns and areas arrive in layer IV. Outputs from layer IV mainly go to layers II and III in the same column, but some outputs go to layer VI. Outputs from layers II/III go to layers V and VI in the same column, and also to the three cortical areas associated with the hippocampus [5]. Outputs from layer V mainly go to the basal ganglia and cerebellum, while outputs from layer VI target the thalamus and other cortical areas and columns [14].
Dendrites are the major submodules of pyramidal neurons, and terminal dendritic branches are the major submodules of dendrites. A terminal dendritic branch detects a group of very similar conditions defined by all the combinations of synaptic weights that can cause the branch to inject voltage deeper into the dendrite. A dendrite detects a group of somewhat less similar conditions defined by all the combinations of branches that together can result in the detection of the pyramidal neuron condition (sometimes called the receptive field of the neuron). Different dendrites detect groups of conditions defined within inputs from different cortical sources [15]. A cortical column defines and detects a group of somewhat similar receptive fields [16]. A cortical area defines and detects a range of receptive fields within the inputs to the area.
Information model for the cortex: Different cortical areas tend to target different regions of the striatum of the basal ganglia [1]. As illustrated in Figure 2, this corresponds with receptive fields defined and detected by different areas being most effective for recommending different types of behaviors. Areas in the orbital and medial prefrontal cortex are effective for recommending strategic behaviors, dorsolateral prefrontal areas for recommending more tactical behaviors, the premotor cortex for recommending more specific behaviors, and the motor cortex for recommending detailed behaviors.
Cortical modules on every level are defined by groups of similar information processes. The more detailed modules define and detect groups of very similar conditions, and the higher-level modules define and detect groups of somewhat less similar conditions. The driving force behind this type of organization is the need to make the most effective use of physical information processing resources. Collecting similar conditions into physical groups minimizes the connectivity required. It is important to note that any cognitive process will require information processes performed by many different modules, and any module will provide processes in support of many different cognitive processes. Hence there will be no correspondences between categories of cognitive processes and modules on any level.
A key role of cortical columns is to provide the information needed by the hippocampal system to manage changes to cortical receptive fields [10]. If receptive field expansions are needed, the expansions with the least undesirable behaviors al side effects will be in neurons that are already close to detecting their fields. Such neurons will tend to be located in columns with strong activity in layers II/III but no layer V/VI activity. Layer II/III activity is therefore communicated to the hippocampal system from columns all across the cortex. As described in more detail below, the hippocampal system uses this information to identify the most appropriate cortical columns for receptive field expansions and generates outputs targeting layer V/VI neurons in those columns.
Thalamus
The thalamus is made up of about 60 nuclei [17], with three or four different types [18]. The bulk of the thalamus is made up of the dorsal nuclei. Each dorsal nucleus is reciprocally connected with one or a group of cortical areas. Some dorsal nuclei are also connected with one of the senses, and these nuclei are sometimes classified as a different type. All dorsal nuclei contain the same types of neurons: the excitatory thalamocortical projection neurons that target cortical pyramidal neurons in layer IV, and inhibitory interneurons that target thalamocortical projection neurons within the same nucleus.
The Thalamic Reticular Nucleus (TRN) is wrapped around the outside of the dorsal nuclei. Each dorsal nucleus is associated with a different region of the TRN, and all the connectivity between the dorsal nucleus and its cortical areas passes through that region and drops connections on to the TRN neurons. All TRN neurons are inhibitory, and when they fire generate sequences of action potentials at a frequency of about 40 Hz, the gamma frequency observed in the EEG. These TRN neurons target the thalamocortical projection neurons.
The other thalamic nuclei are called intralaminar, these nuclei are smaller and are located in the spaces between the dorsal nuclei. The intralaminar nuclei target relatively widely across the brain, but one key connectivity is that they receive inputs from the cerebellar nuclei and target the striatum of the basal ganglia [19], especially the D2 population of neurons in the striatum [20].
Information model for the thalamus: The thalamus releases the sensory or cortical activity selected by the basal ganglia to cortical targets or out of the cortex to drive behaviors. However, on the timescale of such releases, all the connectivity is unchanged, so the question arises: What does “release” mean? When a glutamatergic action potential arrives at a target pyramidal neuron, it injects a postsynaptic potential that rises to a peak in 1-2 milliseconds and then decays with a half-life of about 5 milliseconds [21]. The implication is that unless two action potentials arrive within less than about 5 milliseconds of each other, they will not reinforce each other significantly. Release by the thalamus is achieved by bunching the output action potentials together in time so that they will have a much stronger effect on their targets [8,22].
As shown in Figure 3, there is a positive feedback loop between a dorsal thalamic nucleus and its corresponding cortical area. Thalamocortical projection neurons target cortical pyramidal neurons in layer IV, layer IV pyramidals target layer VI pyramidals via layers II/III, and layer VI pyramidals target back to the thalamocortical neurons. The positive feedback loop is kept in check by constant inhibitive input from the basal ganglia. The basal ganglia signal the selection of a release behaviors by reducing the inhibition of the thalamic nucleus corresponding with that behaviors. This reduction results in an increase in activity in the thalamocortical loop. This increase in activity triggers the inhibitory firing of the TRN interneurons at about 40 Hz (the gamma band frequency observed in the EEG). This limits the increase in activity but imposes a gamma band modulation on the activity in the loop. The gamma band modulation bunches layer VI output action potentials around the peaks in the modulation, effectively releasing them to other cortical areas.
As also shown in Figure 3, excitatory input from the cerebellum targets the thalamic dorsal nucleus. When the cerebellum is controlling a sequence of release behaviors, this cerebellar input implements a release.
Outputs from the basal forebrain target the TRN interneurons [23]. Some of these outputs are excitatory and others inhibitory. These outputs superimpose an 8 Hz modulation (the theta band in the EEG) on the gamma modulation [24], further bunching the output action potentials. This increases the net effect on the target cortical area and drives receptive field expansions when required.
Basal ganglia
As illustrated in Figure 4, the major submodules of the basal ganglia are a set of nuclei. These nuclei are the striatum, the GPi/SNr, the GPe, the STN, and the MDN [25]. The principal neurons in the striatum, the GPi/SNr and the GPe are inhibitory. STN principal neurons are excitatory, and MDN neurons modulate their targets in various ways. The ventral end of the striatum is also called the nucleus accumbens (subdivided into shell and core), and the dorsal end is also called the putamen, with the region between also called the caudate nucleus. The GPi/SNr combines two structures, the globus pallidus internal segment and the substantia nigra pars reticulata, which are the same nucleus but divided by an unrelated band of axons. The dorsal end of the midbrain dopamine neurons (MDN) is also called the substantia nigra pars compacta (SNc) and the ventral end is the ventral tegmental area (VTA).
There is massive excitatory input from layer V cortical pyramidal neurons to medium spiny neurons (MSNs) in the striatum. There is also massive inhibitory output from the GPi/SNr to the dorsal thalamic nuclei. This output is tonic, the neurons constantly fire without any inputs, and therefore constantly inhibit almost the entire thalamus.
MSN outputs from the striatum are inhibitory, and target other nuclei in the basal ganglia. Two different intermingled regions exist in the striatum, known as the matrix and patches. Within the matrix, there are two populations of MSNs, labeled D1 and D2. The D1 MSN population directly targets neurons in the GPi/SNr, inhibits those GPi/SNr neurons, and therefore reduces the inhibition of the thalamic dorsal nuclei. The D2 population targets the GPi/SNr via two intermediary nuclei, the GPe and STN. The STN excites the GPi/SNr and therefore this pathway increases the inhibition of the thalamic dorsal nuclei. An MSN in the D1 population tends to inhibit small subsets of GPi/SNr neurons, while an MSN in the D2 population tends to excite large numbers of GPi/SNr neurons through the STN [26].
The principal neurons of the MDN are dopaminergic. These neurons fire in two different modes. In one mode, called tonic firing, the neuron produces a steady stream of 4-5 action potentials per second. In the other mode, called burst firing, the neuron produces 2–5 spikes at ~15 Hz [27]. The firing is regulated by a complex combination of inputs from various structures including the patches and matrix of the striatum, the GPi/SNr, and the STN [28]. These structures can target both the principal dopaminergic neurons and inhibitory interneurons in the MDN.
In Figure 4, the striatum targets the MDN, and the MDN targets back to the striatum. There is a spiral aspect to this reciprocal connectivity [29] illustrated in Figure 5. The part of the striatum that gets inputs from one part of the MDN includes somewhat more dorsal parts of the striatum than the part that provides outputs to that part of the MDN.
Information model for the basal ganglia: A key behaviors al observation is that MSNs or small groups of MSNs in the striatum actually correspond with specific individual behaviors [30]. The axon of one cortical pyramidal neuron makes one synapse on thousands of different striatal MSNs [31]. In information terms, a pyramidal neuron recommends thousands of different behaviors, and the weight of a cortical pyramidal neuron synapse on to an MSN is the recommendation weight of the detection of the pyramidal neuron receptive field in favor of the behaviors corresponding with the MSN. The output of a D1 population MSN is the total recommendation weight in favor of the corresponding behaviors across all currently detected cortical receptive fields. The output of a D2 population MSN is the total recommendation weight against any behaviors except its corresponding behaviors.
Within the striatum, there is significant inhibition of the D1 population by the D2 population [32]. Neurons in the GPi/SNr also correspond with individual behaviors [33]. D1 population striatal neurons directly target small numbers of GPi/SNr neurons corresponding with similar behaviors and D2 population neurons indirectly target large numbers of GPi/SNr neurons corresponding with different behaviors [26]. The indirect targeting is via the GPe and STN. Hence the D1 population encourages the selection of their corresponding behaviors in the GPi/SNr by inhibiting the neuron corresponding with those behaviors and therefore reducing the inhibition of those behaviors in the thalamus. Indirect targeting discourages the selection of any behaviors other than the corresponding behaviors by exciting the neurons corresponding with those other behaviors. There is thus a competition within both the striatum and GPi/SNr that determines the behaviors with the largest current recommendation strength.
A key requirement is ensuring that multiple incompatible behaviors are not selected at the same time, such as moving the same limb in different directions. On the other hand, it is also important that generally some behaviors are selected. The tonal dopamine firing in the SNc is the mechanism for balancing these requirements. The number of dopaminergic neurons firing tonally in the SNc determines the level of background dopamine in the striatum. The level of background dopamine regulates the relative activity of the D1 and D2 populations: when the background level of dopamine increases, D1 activity increases relative to D2 [34]. If activity in the STN and GPi/SNr indicates that no behaviors are being selected, the number of dopaminergic neurons firing in the tonal mode increases, increasing the chance of a selection by increasing D1 activity relative to D2. If activity in the STN and GPi/SNr indicates that multiple behaviors of the same type are being selected, the number of dopaminergic neurons firing in the tonal mode decreases, decreasing the chance of a selection by decreasing D1 activity relative to D2.
In novel situations, no behaviors may have a strong total recommendation strength. Because of its role in cortical receptive field change management [10], activity in parts of the hippocampus is proportional to the degree of novelty in the current situation [35]. Inputs from the hippocampus to the ventral striatum [36] increase the chance of a behavior selection in novel situations.
Recommendation weight change behaviors must also be selected. When favorable circumstances occur in the course of experience, some cortical receptive fields detected in those circumstances have recommendation strengths in favor of increasing the recommendation strengths that resulted in behaviors that were selected slightly earlier. Other receptive fields recommend decreasing such recommendation strengths. Weight change behaviors are selected by striatal MDNs and implemented by regulation of both the burst and tonal firing of dopaminergic neurons in the MDN. MSNs in the matrix target inhibitory interneurons in the MDN which in turn target dopaminergic neurons [28].
An important constraint is that recommendation strengths cannot be allowed to grow indefinitely. The reason for this constraint is that a receptive field is associated with a range of recommendation strengths. Excessive growth in one of the strengths would mean a loss of the value of all the others. To give an example, suppose some receptive field was often detected when viewing vaguely spherical objects. Such objects could include balls, apples, globes, or heads. If as a result of playing soccer, the recommendation weight of this field in favor of the behaviors of kicking grew without limit, kicking could often be the behavior with the predominant weight when the other types of the object were perceived.
It is observed [37] that when learning new behaviors, burst dopamine firing occurs after a reward. However, once learning is established, the burst firing shifts to the time of the cue for the rewarded behaviors. If the reward does not occur after a previously learned behavior, there is a drop in dopamine firing at the time the reward had previously occurred. Burst firing following behavior is the signal that triggers a long-term increase in the recommendation weights of recently detected receptive fields in favor of the behaviors. The shift of the burst firing to an earlier time means that once learning is established, there will be no further increase in recommendation weights. The burst firing at the time of the cue will slightly increase the level of background dopamine, increasing the chance of a behavior being selected in the near future but in the absence of any recent behaviors will not change any recommendation weights. In other words, it encourages the acceptance of whatever behaviors are most strongly recommended following the cue.
When a behavior is selected, the synaptic weights of the glutamatergic synapses made by currently active cortical pyramidal neurons on to the MSN corresponding with the behaviors are sufficient to make that MSN fire strongly. In this situation, the synaptic weights of all the glutamatergic synapses on the MSN that received an action potential just before the neuron fired are increased [38]. Such increases decline back to their original values over a period of about an hour. Increases to recommendation strengths require that such synaptic weight increases are prolonged long-term. Decreases require decreasing of synaptic weights that increased to below their previous values. Different changes in dopaminergic neuron activity implement these long-term changes [39]. Sufficient recommendation strength in favor of increases in recently used recommendation strengths trigger burst firing of dopaminergic neurons in the MDN that target the striatum. If a burst dopaminergic input is received at a nearby synapse shortly after the glutamatergic synaptic weight increase, the increase is prolonged long term. In information terms, the recommendation weights of the cortical receptive fields that recommended the recent behaviors are increased. Recommendation strengths in favor of decreases trigger a brief drop in the number of dopaminergic neurons firing tonally. The resultant decrease in background dopamine results in a decrease in the weights of recently active synapses onto MSNs that were recently fired.
Rewarding reward behaviors: An important issue is how recommendation strengths in favor of reward behaviors are themselves rewarded. Rewarding reward behaviors will have a very potent effect on future behaviors and must be carefully managed. Cortical receptive fields with such recommendation strengths can initially be defined genetically, but it must be possible for experience to evolve these receptive fields, change their recommendation strengths, and assign such recommendation strengths to other heuristically defined fields. For example, newborns can respond to a smiling face, tending to look at such faces more often than faces with other expressions [40]. This observation can be interpreted as indicating that cortical receptive fields roughly corresponding with the shape of a smiling face can be genetically defined, and such receptive fields are genetically assigned recommendation strengths in favor of reward behaviors. As a result, the behavior of looking towards such a face is rewarded even very soon after birth.
However, more sophisticated reward mechanisms are required which must be defined heuristically, although they can be bootstrapped from the genetically defined mechanisms. One mechanism is that the genetically defined receptive fields detected within visual experiences of smiling faces could be expanded to be detected within circumstances that often occurred at the same time as a smiling face. In this way, circumstances like applause or other indications of approval could acquire recommendation strengths in favor of rewards. However, there must also be a mechanism for changing the weights recommending reward behaviors. To give a specific example to illustrate the issue, consider how the skill of a musician can be evolved. In early learning, receptive fields detected in a smile from a teacher or applause could reward effective muscle movements. On the basis of detection at the same time as these teacher-based receptive fields, many different receptive fields detected within hearing the music as it was played could acquire recommendation strengths in favor of rewarding recent muscle movements. The problem is how to move beyond this. For example, consider the effect of applause at the end of a concert. Such applause would be appropriate for rewarding the strategic behaviors of selecting the time and place of the concert, making a selection of similar behaviors in the future more likely. However, this strategic reward would not be appropriate for rewarding the muscle movements that generated every individual note. Applause at the end of one piece of music would be appropriate for rewarding the tactical behaviors of selecting that piece to play, but not for rewarding every individual muscle movement in the course of playing the piece. However, these strategic and tactical rewards do indicate that overall, the playing was good. The implication is that the receptive fields with recommendation strengths in favor of rewarding muscle movements were effective. In other words, in general, a strategic reward could also reward the behaviors of rewarding tactical behaviors. A tactical reward could also reward the behaviors of rewarding more specific behaviors and so on.
These considerations are implemented by connectivity across the cortex, striatum, and MDN that manages strategic, tactical, specific, and detailed rewards. Cortical conditions detected in orbital and medial prefrontal areas are effective for recommending strategic behaviors. Some conditions in these areas are effective for recommending rewarding strategic behaviors, and those conditions are also effective for recommending rewarding the behaviors of rewarding more tactical behaviors. Recommendations in favor of strategic behaviors and rewarding strategic behaviors target the most ventral part of the striatum, also called the nucleus accumbens shell. Total recommendation strengths in favor of rewarding strategic behaviors are communicated to the most ventral part of the MDN, also called the VTA. Implementation of selected behaviors of rewarding strategic behaviors targets back to the nucleus accumbens shell. However, because those reward behaviors are also effective for rewarding the behaviors of more tactical behaviors, the same part of the MDN also targets the somewhat more dorsal part of the striatum, also called the nucleus accumbens core, where tactical behaviors are selected. Total recommendation strengths in favor of tactical behaviors, rewarding tactical behaviors, and rewarding the behaviors of rewarding more specific behaviors are determined in the somewhat more dorsal part of the striatum (the nucleus accumbens core). Reward behavior selections target a somewhat more dorsal part of the MDN. This part of the MDN targets the nucleus accumbens core, but also a somewhat more dorsal part of the striatum (the caudate nucleus) to implement the behaviors of rewarding the behaviors of rewarding more specific behaviors.
The hippocampal system
In the lower back of the cortex, there are three cortical areas, the entorhinal, parahippocampal, and perirhinal cortices, that are closely associated with the hippocampus. At the edge of these areas, the sheet of pyramidal neurons making up the cortex thins down into a region called the subiculum with fewer layers of pyramidal neurons than the cortex. Beyond the subiculum the cortex-like sheet thins even further into a single layer of pyramidal neurons and curls in a spiral. This spiral is called the CA field and has three regions labeled CA1, CA2, and CA3. The CA fields end inside a wedge of neurons called the dentate gyrus, which is made up of mossy cells and granule cells. The CA fields and dentate gyrus together make up the hippocampus proper [41].
As illustrated in Figure 6, all cortical areas except the primary sensory areas are reciprocally connected with the hippocampus through the entorhinal cortex, directly or via one of the other two cortical areas [42]. Inputs to the hippocampus are derived from cortical layers II and III, and hippocampal outputs target cortical layers V and VI [41].
As shown in Figure 7, layer II/III outputs from the entorhinal cortex to the hippocampus proper directly target pyramidal neurons in the three CA fields and also the mossy cells in the dentate gyrus. Within the hippocampus proper there are two positive feedback loops. In the dentate gyrus granule cells excite mossy cells [43] and mossy cells excite granule cells [44]. Pyramidal neurons in CA3 excite large numbers of other CA3 pyramidal neurons [45]. These two positive feedback loops are linked together. CA3 pyramidals get excitatory inputs from a small number of granule cells and a large number of inputs from inhibitory interneurons [46]. These inhibitory interneurons are themselves targeted by granule cells. With low levels of granule cell activity, excitation of CA3 predominates over inhibition, but as granule cell activity increases, inhibition comes to predominate over excitation [47].
The wider hippocampal system includes reciprocal connectivity with subnuclei in a number of other subcortical nuclei. These nuclei include the anterior nucleus of the thalamus [48], the mammillary bodies of the hypothalamus [49], the basolateral nucleus of the amygdala [41], and the septal nucleus of the basal forebrain [50]. The main routes of this connectivity are illustrated in Figure 8. Physical damage to any of these structures or the connectivity between them and the hippocampus proper results in cognitive deficits similar to those that result from damage to the hippocampus itself [10].
Information model for the hippocampal system: The primary role of the hippocampal system is to determine the cortical pyramidal neuron receptive fields that will expand in response to circumstances with some degree of novelty [10]. The expansions are required to bring the ranges of behaviors and recommendations up to a minimum level, and the number of expansions will be proportional to the degree of novelty. Because there is some degree of novelty in almost all situations, there are almost always some expansions.
To minimize undesirable behavioral side effects, the receptive fields selected for expansion meet two criteria. One is that only a fairly small expansion is needed for the receptive field to be detected. The other is that selected receptive fields will often have expanded in the past at the same time as significant numbers of receptive fields that are already being detected. Receptive fields close to being detected are identified by being in cortical columns with strong layer II/III activity. To achieve the second criterion, receptive fields in the hippocampal cortices correspond with groups of cortical columns that often expanded their receptive fields at similar times in the past. Receptive fields in the hippocampus properly correspond with groups of groups of cortical columns that often expanded their receptive fields at similar times in the past. Hence, as a result, active inputs from the entorhinal cortex correspond with layer II/III activity in groups of cortical columns. Activity in pyramidal neurons in CA3 and granule cells in the dentate gyrus correspond with activity in groups of groups of cortical columns.
The selection process occurs in the structures illustrated in Figure 7, with CA1 outputs driving receptive field expansions in the selected groups of groups of columns. An input to the hippocampus proper from the entorhinal cortex indicates the degree of internal activity in a group of columns across the cortex that tended to expand receptive fields at similar times in the past. If there is a very low degree of novelty in the current circumstances, there will be strong activity in all the entorhinal inputs. There will therefore be strong activity in all the dentate gyrus granule cells, and inhibition of CA3 will predominate over excitation. In other words, CA3 will be shut down and will produce no outputs to CA1, which in turn will result in no outputs to drive expansions. If on the other hand there is some degree of novelty in the current circumstances, some of the inputs from the entorhinal cortex will be lower. Granule cells with receptive fields corresponding with groups of groups of columns in the cortical areas with this lower activity will be lower, allowing CA3 pyramidal neurons with similar receptive fields to build activity. The degree of activity in CA3 will therefore be proportional to the degree of novelty experienced in different cortical regions and will cause CA1 to drive expansions in those regions.
The CA1 selections of groups of groups of columns are decoded through the subiculum, entorhinal cortex, and the other two hippocampal system cortices so that inactive columns that form parts of many of the selected groups of groups are targetted for expansion.
In Figure 8, the anterior thalamus performs the standard thalamic process of releasing pyramidal neuron activity to targets. While the competition within CA3 and the dentate gyrus is going on, there is activity in CA3 that will drive activity in CA1, but this activity is not a good guide to required receptive field expansions. The role of the septal nuclei is to release the CA1/CA3 activity once the selection process in the dentate gyrus and CA3 has been completed. This release is achieved by imposing a 4 Hz (theta band) modulation on top of the 40 Hz (gamma band) modulation, which bunches neuron output spikes even more closely together. The amygdala and hypothalamus influence receptive field expansions in favor of the types of behaviors currently prioritized by the brain [10].
Episodic memory depends on cortical neurons indirectly activating other neurons on the basis of simultaneous activity during a past period of receptive field expansion [51]. Episodic memory therefore requires neuron receptive fields that record this type of temporally correlated activity. Because of the primary role of the hippocampal system, such records are easily established in that system [11]. Semantic memory depends on cortical neurons indirectly activating other neurons on the basis of frequent past simultaneous activity [51]. The receptive fields recording this type of temporally correlated activity are established in the regular cortex. Furthermore, if episodic memory is often recalled, the basis for recall could shift to frequent past simultaneous activity, removing the dependence on the hippocampal system.
The cerebellar system
The structures and major connectivity paths of the cerebellar system have been extensively studied, and are illustrated in Figure 9. The system includes the cerebellar cortex, the cerebellar nuclei, and two nuclei in the brainstem: the pontine nucleus and the inferior olive.
The cerebellum proper is in the lower back of the brain, connected to the pontine and inferior olive nuclei in the brainstem by a thick axon bundle. These two nuclei get massive inputs from the cerebral cortex. The cerebellar cortex is made up of two layers of neurons: an outer layer of large Purkinje neurons; and an inner layer made up of huge numbers of very small neurons called granule cells. Each granule cell gets inputs from a small number of pontine nucleus neurons. Purkinje neurons get inputs from an extremely large number of granule cells. A Purkinje receptive field is therefore defined by very complex combinations of very large numbers of cerebral cortex inputs. A Purkinje cell also gets a large number of synapses from one inferior olive neuron. Outputs from Purkinje cells are inhibitory and target neurons in the cerebellar nuclei. The cerebellar nuclei contain both excitatory and inhibitory neurons. The inhibitory cerebellar nuclei neurons target the inferior olive, and the excitatory neurons provide the outputs from the cerebellar system, targeting the spinal cord and also the thalamus.
At a more detailed level, the cerebellar system is divided into microzones [52,53]. As illustrated in Figure 10, each microzone is made up of a group of Purkinje neurons, neurons in the cerebellar nuclei, and neurons in the inferior olive. With the exception of the excitatory cerebellar neurons, the neurons in these groups mainly target other neurons in the same microzone. There are many thousands of such cerebellar microzones in the brain.
There is also connectivity between the cerebellar system and the basal ganglia [20] as illustrated in Figure 11. The STN targets the cerebellar cortex via the pontine nucleus, and the cerebellar nuclei target the D2 population of MSNs in the striatum via the intralaminar nuclei of the thalamus.
Information model for the cerebellar system: The cerebellar system takes over control of sequences of behaviors that are often used and have previously been learned between the cortex and basal ganglia. Such sequences include muscle movements to maintain posture and balance; muscle movements required for motor behaviors like walking, running, riding a bicycle, playing an instrument, or speaking a language; and sequences of information releases between cortical areas required for thinking. Once transferred to the cerebellum, a sequence can be selected by the basal ganglia but then executed with no further processing by the basal ganglia. As a result, sequences are executed much faster.
One microzone corresponds with one such sequence, and each excitatory cerebellar nuclei neuron corresponds with one behavior in the sequence. Each Purkinje develops a receptive field corresponding with the time when one behavior in the sequence should be initiated. This receptive field is initially defined by huge combinations of cortical receptive fields that happened to be active when the behaviors were initiated under basal ganglia control. When a Purkinje fires, it inhibits all the cerebellar nuclei neurons in its microzone except the one corresponding with its behaviors. The firing of the sequence of Purkinje therefore initiates the sequence of behaviors in the right order. In the case of motor behaviors, the cerebellar outputs target the spinal cord. In the case of cognitive behaviors, cerebellar outputs target the thalamus.
One neuron in the inferior olive targets just the Purkinje corresponding with one behavior. Inferior olive firing close in time to Purkinje firing can induce both long-term increases and long-term decreases in recently active granule cell-Purkinje synapses [54], and the relative timing of Purkinje and inferior olive firing determines the change that takes place. In some cases, strong changes are induced if there is a time difference of about 120 milliseconds [55].
The firing of a Purkinje cell releases the activity of both the excitatory and inhibitory cerebellar nuclei neurons corresponding with the behaviors. The excitatory output drives the behaviors and the inhibitory output blocks the firing of the inferior olive. If the inferior olive firing is not blocked, the information meaning is that the timing of the behaviors was incorrect. The inferior olive firing then shifts the receptive field of the Purkinje in favor of granule cell inputs that occurred at a slightly different time, adjusting the future timing of the behaviors.
Such timing adjustments are the only form of learning available to the cerebellum. Any changes to the order of the behaviors in the sequence or to the actual behaviors require that detailed control reverts to the cerebral cortex and basal ganglia.
The links between the cerebellum and basal ganglia illustrated in Figure 11 ensure that behaviors and control are not asserted by both structures at the same time. When a behavior sequence has been selected by the basal ganglia, excitatory outputs from the cerebellar nuclei indicate that the sequence is being driven. Via the intralaminar these outputs target the D2 neuron population in the striatum of the basal ganglia. The activity of this population inhibits any behavior selection by the basal ganglia while the cerebellar-controlled sequence is under way. Strong excitatory outputs from the STN indicate that there is no new behaviors selection by the basal ganglia, these outputs target the cerebellar cortex via the pontine nucleus and encourage the continuation of the cerebellar behaviors sequence currently under way. Cortical condition detections detected shortly after the end of a sequence indicate successful or unsuccessful completion. These conditions recommend dopamine signals from the MDN to the cerebellar cortex that modulate the conditions recently detected by Purkinje neurons.
Accounting for the deficits resulting from degeneration and damage
The information processes in cortical and subcortical structures described in the previous section can be used to understand motor and cognitive deficits in terms of local damage to subcortical structures. The loss of the information processes performed by the damaged structures results in deficits. Eight types of damage will be discussed.
Parkinson’s disease
The most disabling symptom of Parkinson’s disease is slowness of movement [56]. This manifests itself as a difficulty in performing voluntary movements, initially fine motor tasks like doing up buttons, progressing to movements like getting up from a chair, and eventually to loss of most ability to initiate movement. The anatomical cause of the disease is the loss of SNr dopaminergic neurons and the consequent drop in the concentration of dopamine in the striatum [57]. Symptoms appear when 50% of nigral neurons and 80% of striatal dopamine is lost. Cognitive deficits, for example in planning and memory, are also common [58].
Information model for Parkinson’s disease: The drop in dopamine concentration in the striatum resulting from the loss of dopaminergic neurons in the MDN results in higher activity by the D2 population of MSNs relative to the D1 MSNs. The D1 population recommends in favor of behaviors and the D2 population recommends against behaviors. The healthy dopamine concentration ensures that in most situations a behavior is selected, but not multiple incompatible behaviors. If the dopamine concentration drops in the dorsal striatum, the probability of any voluntary motor behaviors being selected is reduced, leading to the classical symptoms of Parkinson’s disease. If the dopamine concentration is reduced in the somewhat more ventral striatum, the probability of information releases between cortical areas required for cognitive processes is reduced, resulting in the observed cognitive deficits.
Huntington’s disease
The characteristic symptom of Huntington’s disease is rapid uncontrollable muscle movements, leading to a lack of coordination and the inability to sustain voluntary motor movements. Cognitive symptoms include loss of planning ability and difficulties with the acquisition of new motor skills [59]. The anatomical cause of the disease is the loss of MSNs in the striatum, especially the D2 population neurons that project to the GPe.
Information model for Huntington’s disease: A cortical receptive field detection recommends a wide range of behaviors. Hence in response to a population of receptive field detections in the motor and supplementary motor cortices, there will be some total recommendation strength in favor of many different motor behaviors. The D2 MSNs in the striatum recommend against behaviors and prevent the selection of multiple incompatible behaviors. Loss of these neurons means that sometimes multiple incompatible behaviors can be selected at the same time, indicated by the strong firing of multiple MSNs. An example might be moving a limb in two different directions at the same time. Movements like this are not physically possible, and the conflict is resolved in downstream processing in the brainstem and spinal cord. However, the ability of these structures to resolve these conflicts in an integrated fashion is limited, resulting in the observed difficulty in sustaining coordinated voluntary movements because of the insertion of unrelated muscle movements.
Hemiballism
Hemiballism involves sudden violent involuntary limb movements that are inserted into voluntary movements like walking [60]. These involuntary movements occur much less frequently when the patient is at rest, and do not occur during sleep.
Hemiballism generally occurs following a stroke. The classical cause is a stroke damaging the STN, but strokes damaging other locations in the basal ganglia, the thalamus, or even the cortex or cortical white matter can cause similar symptoms in some cases. However, it remains clear that hemiballism does follow STN damage [61] and it is possible that the symptoms are more severe with STN damage [60].
Information model for Hemiballism: Total recommendation strengths against behaviors are determined by the D2 neuron population in the striatum and communicated to the GPi/SNr via the GPe and STN. The STN neurons excite the GPi/SNr neurons, increasing their inhibition of the thalamus and therefore blocking behaviors. Damage to the STN neurons therefore allows the possible selection of multiple incompatible behaviors, which must be resolved closer to muscle control and therefore leads to unselected muscle movements being inserted into voluntary movements. If there are no cortical receptive field detections recommending muscle movements, such as when the patient is at rest, there will be no recommendation strengths in favor of unselected movements. Hence involuntary movements are less common at rest.
Tourette’s syndrome
The characteristic symptom of Tourette’s syndrome is the appearance of sudden, stereotyped physical or vocal actions called tics into ongoing behaviors [62]. These actions can range from relatively simple eye rolling or grunting to more complex clusters of movements like touching objects or saying random phrases. The more complex tics can often be temporarily suppressed by the patient, but if this is done the pressure to perform the tic builds until it is irresistible.
Postmortem studies have failed to identify a clear physical cause for Tourette’s [63], but the most effective treatments for suppressing tics are drugs that block D2 receptors for dopamine [64]. Drugs with a stronger blocking effect like haloperidol are more effective in reducing tics [65]. However, these drugs have side effects including involuntary muscle contractions, uncontrolled jerky movements, and inability to sit still [66,67]. Side effects also include cognitive dulling and mood swings.
Information model for Tourette’s syndrome: Tics are sequences of detailed muscle movements that frequently occur. Hence such tics will be programmed in and controlled by the cerebellar system. Transfer of control to the cerebellar system from the basal ganglia is maintained when excitatory outputs from the STN target the cerebellar cortex via the pontine nucleus and excitatory outputs from the cerebellar nuclei target the D2 population of MSNs in the striatum via the thalamic intralaminar nuclei.
Hence in Tourette’s syndrome, the cerebellum records behaviors ally irrelevant sequences, and some cortical receptive fields acquire excessive recommendation strengths in favor of these sequences. The reason that dopamine antagonists targeting D2 receptors weaken the tics is that they weaken activity in the cerebellar-basal ganglia links that maintain the tics. Reduced activity of the D2 population in the striatum reduces the activity in the STN and therefore the outputs from the STN encourage the performance of the tic.
The problem with this approach to treatment is that reducing D2 population activity also reduces the recommendation strengths against behaviors, resulting in the selection of multiple incompatible behaviors. Hence the irrelevant jerky movements that result from the drugs. In addition, blocking of D2 population activity in regions of the striatum that select internal cortical release behaviors results in sudden changes in these internal releases that are experienced as sudden shifts in mood.
Thalamic strokes
Each dorsal thalamic nucleus is heavily interconnected with one or a group of cortical areas. Stroke damage to the thalamus can result in a wide range of different behaviors al deficits, but almost all thalamic deficits mimic the type of deficit resulting from damage to the cortical areas with which the thalamus is interconnected [68].
Information model for thalamic strokes: Thalamic damage to one dorsal nucleus affects the release of information from the cortical areas interconnected with that nucleus, and will therefore result in attention, cognitive, or motor deficits similar to those resulting from damage to the interconnected cortical areas.
Cerebellar lesions
The characteristic symptoms of cerebellar lesions are called ataxias. These are jerky and inaccurate limb or eye movements, or problems with the muscle movements to maintain body tone or balance [69]. A patient with damage to their left cerebellar hemisphere affecting their right arm described their symptoms as “the movements of my left arm are done subconsciously, but I have to think out each movement of the right arm. I come to a dead stop in turning and have to think before I start again” [70]. Cerebellar damage can also affect the putting together of syllable strings to generate speech [71]. There is also evidence that cerebellar damage can result in poorer performance of some cognitive tasks [72]. However, it is notable that significant recovery from cerebellar ataxias is possible, with recovery being aided by physiotherapy targeting the movement problem area [73].
Information model for cerebellar lesions: The cerebellum records sequences of behaviors previously learned between the cortex and basal ganglia, and when the sequence is selected by the basal ganglia, the cerebellum rapidly and accurately executes the sequence. Behaviors in the sequence do not have to be selected individually by the cerebellum. Cerebellar damage results in the loss of the records of some sequences. As a result, control shifts back to the basal ganglia, with each behavior requiring individual selection on the basis of cortical inputs. These sequences therefore become much slower and less accurate, and individual selection is experienced as the need to think out each movement. However, it is possible for the sequence to be recorded again in an undamaged part of the cerebellum, resulting in recovery.
Drug addiction behaviors
Drug addiction, or substance use disorder, is a condition in which a person is focused on using a certain substance to the detriment of their day-to-day life [74]. Addictive drugs are subjectively pleasurable. Addiction starts with occasional use and progresses to habitual compulsive drug-seeking behaviors. Once established, a key trigger for drug-seeking is exposure to general environmental cues that have become associated with drug use. Complete removal of these cues can result in drug-seeking behaviors no longer occurring [75].
All of the approximately 100 chemicals able to cause compulsive drug use in humans are directly or indirectly dopamine agonists [74]. In particular, they appear to act on the dopamine link between the ventral MDN (or VTA) and the ventral striatum (or nucleus accumbens) [74].
Information model for drug addiction behaviors: The ventral striatum selects strategic behaviors, and burst firing of dopaminergic neurons in the ventral MDN increases the weight of the recommendation strengths that resulted in recently implemented strategic behaviors. The dopaminergic neuron activity following drug taking therefore increases the recommendation strengths of recently detected cortical receptive fields in favor of the strategic behaviors selection that resulted in drug seeking. This strategic behaviors was the release of the receptive fields that strongly recommend drug seeking behaviors to cortical areas that are effective for recommending more tactical behaviors. The receptive fields detected in these areas will therefore recommend different more tactical behaviors of the drug-seeking type. Acceptance of one of these recommended tactical behaviors is implemented by the release of the receptive fields that recommended it to cortical areas effective for recommending more specific behaviors and so on.
The receptive fields recommending strategic drug-seeking behaviors are complex combinations of environmental cues. Hence as long as these cues occur in the environment, receptive fields with large recommendation strengths in favor of drug-seeking behaviors will be detected, and their detection will drive the selection of more and more specific drug-seeking behaviors, leading to the use of the drug. If these cues are no longer present in the environment, the receptive fields with large recommendation strengths in favor of drug-seeking will no longer be detected, and drug-seeking will no longer occur. Thus American soldiers who became addicted to drugs in Vietnam no longer exhibited drug-seeking behaviors after their return to America [75].
Hippocampal system damage
Damage to the hippocampal system results in a range of deficits that have been extensively investigated [6]. The most obvious deficit is amnesia, the loss of the ability to create new memories for facts, words, people, or events. It remains possible to recall semantic memories created before the damage, for example, the patient retains knowledge of prior vocabulary but cannot learn new words. In the case of episodic memory, events in the few years preceding damage cannot be recalled, but some events more remote in time can be recalled.
Amnesic patients can learn new simple motor skills. For example, such patients can acquire the skill of tracing a complex figure while only viewing the figure and hands in a mirror [6]. This skill is difficult to learn even for a normal subject. Amnesic patients improve over a number of sessions, but each time has no memory of earlier sessions. Complex motor skills requiring new declarative-type information cannot be acquired. However, complex skills acquired before hippocampal damage are retained.
Epilepsy is the symptom of excessive neuron activity in some regions of the cortex [76]. This excess activity is triggered from some focal point, and the hippocampus proper is often that focal point [77].
Information model for hippocampal system damage: Hippocampal system damage results in loss of the ability to make changes to cortical receptive fields. Such changes are the basis for new semantic and episodic memories, and the creation of such memories is therefore not possible. However, all the existing cortical receptive fields are as previously defined, and all their recommendation strengths in the basal ganglia remain. All the very complex receptive fields in the cerebellum that drive previously learned sequences of behaviors are also unaffected. Hence previously learned skills are retained. If a skill can be acquired through cortical receptive fields as previously defined acquiring new recommendation strengths in the basal ganglia, such acquisition is possible. However, if a new skill requires changes to cortical receptive fields, such a skill cannot be successfully acquired.
Recall of episodic memories generally relies on pyramidal neuron receptive fields in the hippocampal system that record groups of cortical neurons active during past periods of receptive field expansion. Damage to the hippocampal system blocks retrievals on this basis. However, retrieval of memories that were often retrieved prior to the hippocampal system damage may have become based on frequent past simultaneous neuron activity, and such retrievals will still be possible. Generally, such memories will be of events long before the damage.
The connected positive feedback loops within the hippocampus properly can easily be a focus for epileptic seizures. Because of the importance of the cortical change management function performed by the hippocampal system and the need for these loops to perform that function, they have been retained despite natural selection pressures to reduce the risk of epileptic seizures.
Cortical damage
The deficits that result from local damage to the cortex are generally less easily specified than in the subcortical structures. For example, it is found that executive functions like verbal fluency, matching patterns, and managing attention are not specifically associated with any one cortical area. Rather, any one function is measurably degraded by damage to a wide range of areas, As Henry and Crawford [78] comment: “Both frontal and non-frontal brain regions are necessary for intact executive functions ..... sensitivity [is] fairly robust and reliable among commonly used tests of executive function, yet specificity is modest at best”.
Information model for cortical damage: Each cortical area defines and detects conditions within a different range of complexity. Each condition recommends a wide range of different behaviors. Anyone’s behaviors may be usefully recommended by conditions detected within many different areas. Damage to one area will remove some but not all of the recommendation strengths in favor of any one behavior or type of behavior. Local damage will therefore tend to remove some of the recommendation’s strengths in favor of a wide range of behaviors, but not all of the strength in favor of any one behaviors.
Complications at more detailed levels
The requirement that information processing resources be organized in a modular hierarchy based on information process similarity discussed in section 2 is not absolute. There are also operational requirements, such as the need to make very rapid responses to some environmental situations. Such operational requirements may conflict with the need to minimize resources, and natural selection pressures will result in some compromises. Such compromises may involve the inclusion of somewhat less similar information processes in a module and/or additional information exchange between modules. These compromises will show up as additional submodules within a module and additional connectivity between modules. For example, although Figure 4 is a good representation of the major nuclei and connectivity within the basal ganglia, there are other more detailed nuclei and minor connectivity paths that exist [79].
These factors are a major reason why descriptions at high levels are approximate. They make the more detailed levels of the hierarchy of descriptions more complex. However, the ability to map between levels means that the high-level descriptions are an effective basis for achieving understanding.
Conclusion
An approach based on theoretical demonstrations of the architectural constraints on brains makes it possible to identify the different types of information processes performed by different local anatomical circuits. These different types of information processes combine to generate motor and cognitive behaviors. Damage to a specific anatomical structure removes the type of information process performed by that structure and removal of one type results in specific motor or cognitive deficits. The deficits that occur in Parkinson’s disease, Huntingdon’s disease, Hemiballism, Tourette’s syndrome, hippocampal system damage, and strokes affecting the thalamus or cerebellum can therefore be understood in terms of the loss of different types of information processes performed within the cortex-hippocampus-thalamus-basal ganglia-cerebellum system.
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-81. doi: 10.1146/annurev.ne.09.030186.002041. PMID: 3085570.
- Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013 May;17(5):241-54. doi: 10.1016/j.tics.2013.03.003. Epub 2013 Apr 9. PMID: 23579055; PMCID: PMC3645327.
- Middleton FA, Strick PL. Cerebellar output: motor and cognitive channels. Trends Cogn Sci. 1998 Sep 1;2(9):348-54. doi: 10.1016/s1364-6613(98)01220-0. PMID: 21227231.
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008 Sep;44(8):1037-66. doi: 10.1016/j.cortex.2008.04.004. Epub 2008 May 23. PMID: 18614161; PMCID: PMC3738020.
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994 Dec 22;350(4):497-533. doi: 10.1002/cne.903500402. PMID: 7890828.
- Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009 Jan 15;61(1):6-9. doi: 10.1016/j.neuron.2008.12.023. PMID: 19146808; PMCID: PMC2649674.
- Coward LA. The Recommendation Architecture: lessons from the design of large scale electronic systems for cognitive science. Cogn. Syst. Res. 2001; 2(2): 111-156.
- Coward LA. Towards a Theoretical Neuroscience: from Cell Chemistry to Cognition. Amsterdam: Springer. 2013.
- Coward LA. Hierarchies of description enable understanding of cognitive phenomena in terms of neuron activity. Cogn Process. 2024 Mar 14. doi: 10.1007/s10339-024-01181-5. Epub ahead of print. PMID: 38483738.
- Coward LA. The hippocampal system as the cortical resource manager: a model connecting psychology, anatomy and physiology. Adv Exp Med Biol. 2010;657:315-64. doi: 10.1007/978-0-387-79100-5_18. PMID: 20020356.
- Coward LA, Gedeon TD. Using the Change Manager Model for the Hippocampal System to Predict Connectivity and Neurophysiological Parameters in the Perirhinal Cortex. Comput Intell Neurosci. 2016;2016:8625875. doi: 10.1155/2016/8625875. Epub 2015 Dec 27. PMID: 26819594; PMCID: PMC4706880.
- Amunts K, Zilles K. Architectonic Mapping of the Human Brain beyond Brodmann. Neuron. 2015 Dec 16;88(6):1086-1107. doi: 10.1016/j.neuron.2015.12.001. PMID: 26687219.
- Mountcastle VB. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408-34. doi: 10.1152/jn.1957.20.4.408. PMID: 13439410.
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010 Mar 31;4:13. doi: 10.3389/fnana.2010.00013. PMID: 20556241; PMCID: PMC2885865.
- Spratling MW. Cortical region interactions and the functional role of apical dendrites. Behav Cogn Neurosci Rev. 2002 Sep;1(3):219-28. doi: 10.1177/1534582302001003003. PMID: 17715594.
- Tanaka K. Columns for complex visual object features in the inferotemporal cortex: clustering of cells with similar but slightly different stimulus selectivities. Cereb Cortex. 2003 Jan;13(1):90-9. doi: 10.1093/cercor/13.1.90. PMID: 12466220.
- Cassel JC, de Vasconcelos AP. The cognitive thalamus: A bridal chamber not to forget. Neurosci Biobehav Rev. 2015 Jul;54:1-2. doi: 10.1016/j.neubiorev.2015.01.017. Epub 2015 Jan 20. PMID: 25616184.
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015 Jul;54:89-107. doi: 10.1016/j.neubiorev.2015.01.014. Epub 2015 Jan 20. PMID: 25616182; PMCID: PMC4976455.
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002 Sep;39(2-3):107-40. doi: 10.1016/s0165-0173(02)00181-9. PMID: 12423763.
- Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010 Sep;20(3):261-70. doi: 10.1007/s11065-010-9143-9. Epub 2010 Sep 3. PMID: 20811947; PMCID: PMC3325093.
- Jonas P. The Time Course of Signaling at Central Glutamatergic Synapses. News Physiol Sci. 2000 Apr;15:83-89. doi: 10.1152/physiologyonline.2000.15.2.83. PMID: 11390884.
- Coward LA. Simulation of a Proposed Binding Model, in Brain Inspired Cognitive Systems 2004, ed. Smith LS, Hussain A, Aleksander I. Stirling: University of Stirling. 2004.
- Agostinelli LJ, Geerling JC, Scammell TE. Basal forebrain subcortical projections. Brain Struct Funct. 2019 Apr;224(3):1097-1117. doi: 10.1007/s00429-018-01820-6. Epub 2019 Jan 5. PMID: 30612231; PMCID: PMC6500474.
- Tingley D, Alexander AS, Quinn LK, Chiba AA, Nitz D. Multiplexed oscillations and phase rate coding in the basal forebrain. Sci Adv. 2018 Aug 1;4(8):eaar3230. doi: 10.1126/sciadv.aar3230. PMID: 30083600; PMCID: PMC6070333.
- Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012 Dec 1;2(12):a009621. doi: 10.1101/cshperspect.a009621. PMID: 23071379; PMCID: PMC3543080.
- Hazrati LN, Parent A. Striatal and subthalamic afferents to the primate pallidum: interactions between two opposite chemospecific neuronal systems. Prog Brain Res. 1993;99:89-104. doi: 10.1016/s0079-6123(08)61340-0. PMID: 7509082.
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984 Nov;4(11):2877-90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. PMID: 6150071; PMCID: PMC6564720.
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012 Jun 7;74(5):858-73. doi: 10.1016/j.neuron.2012.03.017. PMID: 22681690.
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000 Mar 15;20(6):2369-82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. PMID: 10704511; PMCID: PMC6772499.
- West MO, Carelli RM, Pomerantz M, Cohen SM, Gardner JP, Chapin JK, Woodward DJ. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J Neurophysiol. 1990 Oct;64(4):1233-46. doi: 10.1152/jn.1990.64.4.1233. PMID: 2258744.
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998 Jun 15;18(12):4722-31. doi: 10.1523/JNEUROSCI.18-12-04722.1998. PMID: 9614246; PMCID: PMC6792707.
- Bahuguna J, Aertsen A, Kumar A. Existence and control of Go/No-Go decision transition threshold in the striatum. PLoS Comput Biol. 2015 Apr 24;11(4):e1004233. doi: 10.1371/journal.pcbi.1004233. PMID: 25910230; PMCID: PMC4409064.
- Gdowski MJ, Miller LE, Parrish T, Nenonene EK, Houk JC. Context dependency in the globus pallidus internal segment during targeted arm movements. J Neurophysiol. 2001 Feb;85(2):998-1004. doi: 10.1152/jn.2001.85.2.998. PMID: 11160530.
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011 Mar;63(1):182-217. doi: 10.1124/pr.110.002642. Epub 2011 Feb 8. PMID: 21303898.
- Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014 Jul;24(7):773-83. doi: 10.1002/hipo.22268. Epub 2014 Mar 21. PMID: 24596296; PMCID: PMC4065199.
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999 Jun 29;877:49-63. doi: 10.1111/j.1749-6632.1999.tb09260.x. PMID: 10415642.
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005 Jun 29;25(26):6235-42. doi: 10.1523/JNEUROSCI.1478-05.2005. PMID: 15987953; PMCID: PMC6725057.
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998 Dec 15;18(24):10464-72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. PMID: 9852584; PMCID: PMC6793365.
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000 Mar;25(3):515-32. doi: 10.1016/s0896-6273(00)81056-9. PMID: 10774721.
- Farroni T, Menon E, Rigato S, Johnson MH. The perception of facial expressions in newborns. Eur J Dev Psychol. 2007 Mar;4(1):2-13. doi: 10.1080/17405620601046832. Epub 2007 May 3. PMID: 20228970; PMCID: PMC2836746.
- Insausti R, Amaral DG. Hippocampal formation, in The Human Nervous System, ed. G. Paxinos (San Diego, CA: Elsevier Academic Press). 2004; 871-914.
- Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: organization of cortical inputs and interconnections with amygdala and striatum. Semin Neurosci. 1996; 8: 3-12.
- Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985 Oct;14(5):835-57. doi: 10.1007/BF01170832. PMID: 2419523.
- Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992 Oct;2(4):349-62. doi: 10.1002/hipo.450020403. PMID: 1284975.
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1-11. doi: 10.1016/s0079-6123(08)61237-6. PMID: 2203093.
- Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998 May 1;18(9):3386-403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. PMID: 9547246; PMCID: PMC6792657.
- Bragin A, Jandó G, Nádasdy Z, van Landeghem M, Buzsáki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol. 1995 Apr;73(4):1691-705. doi: 10.1152/jn.1995.73.4.1691. PMID: 7643175.
- Shibata H. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J Comp Neurol. 1993 Nov 15;337(3):431-45. doi: 10.1002/cne.903370307. PMID: 7506716.
- Allen GV, Hopkins DA. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J Comp Neurol. 1989 Aug 15;286(3):311-36. doi: 10.1002/cne.902860303. PMID: 2504784.
- Colom LV. Septal networks: relevance to theta rhythm, epilepsy and Alzheimer's disease. J Neurochem. 2006 Feb;96(3):609-23. doi: 10.1111/j.1471-4159.2005.03630.x. Epub 2006 Jan 9. PMID: 16405497.
- Coward LA. Modelling Memory and Learning Consistently from Psychology to Physiology, in Perception-Action Cycle: Models, Architectures, and Hardware, ed. V. Cutsuridis, H. Amir, and J. Taylor (Amsterdam: Springer). 2011; 52-123.
- Oscarsson O. Functional units of the cerebellum – sagittal zones and microzones. Trends Neurosci. 1979; 2: 143-145.
- Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, Ebner TJ, Isope P, Jörntell H, Lackey EP, Lawrenson C, Lumb B, Schonewille M, Sillitoe RV, Spaeth L, Sugihara I, Valera A, Voogd J, Wylie DR, Ruigrok TJH. Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]. Cerebellum. 2018 Oct;17(5):654-682. doi: 10.1007/s12311-018-0952-3. Erratum in: Cerebellum. 2018 Jun 21;: PMID: 29876802; PMCID: PMC6132822.
- D'Angelo E. The organization of plasticity in the cerebellar cortex: from synapses to control. Prog Brain Res. 2014;210:31-58. doi: 10.1016/B978-0-444-63356-9.00002-9. PMID: 24916288.
- Suvrathan A, Payne HL, Raymond JL. Timing Rules for Synaptic Plasticity Matched to Behavioral Function. Neuron. 2016 Dec 7;92(5):959-967. doi: 10.1016/j.neuron.2016.10.022. Epub 2016 Nov 10. Erratum in: Neuron. 2018 Jan 3;97(1):248-250. PMID: 27839999; PMCID: PMC5165237.
- Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004 May 29;363(9423):1783-93. doi: 10.1016/S0140-6736(04)16305-8. PMID: 15172778.
- Marsden CD. Parkinson's disease. Lancet. 1990 Apr 21;335(8695):948-52. doi: 10.1016/0140-6736(90)91006-v. PMID: 1691427.
- Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. J Neurol. 1997 Jan;244(1):2-8. doi: 10.1007/pl00007725. PMID: 9007738.
- Walker FO. Huntingdon’s disease. Lancet. 2007; 369: 218-228.
- Postuma RB, Lang AE. Hemiballism: revisiting a classic disorder. Lancet Neurol. 2003 Nov;2(11):661-8. doi: 10.1016/s1474-4422(03)00554-4. PMID: 14572734.
- Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005 Aug;76(6):393-413. doi: 10.1016/j.pneurobio.2005.09.005. Epub 2005 Oct 24. PMID: 16249050.
- Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, Frith CD, Frackowiak RS, Dolan RJ. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000 Aug;57(8):741-8. doi: 10.1001/archpsyc.57.8.741. PMID: 10920461.
- Singer HS, Minzer K. Neurobiology of Tourette's syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain Dev. 2003 Dec;25 Suppl 1:S70-84. doi: 10.1016/s0387-7604(03)90012-x. PMID: 14980376.
- Eddy CM, Rickards HE, Cavanna AE. Treatment strategies for tics in Tourette syndrome. Ther Adv Neurol Disord. 2011 Jan;4(1):25-45. doi: 10.1177/1756285610390261. PMID: 21339906; PMCID: PMC3036957.
- Scahill L, Erenberg G, Berlin CM Jr, Budman C, Coffey BJ, Jankovic J, Kiessling L, King RA, Kurlan R, Lang A, Mink J, Murphy T, Zinner S, Walkup J; Tourette Syndrome Association Medical Advisory Board: Practice Committee. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006 Apr;3(2):192-206. doi: 10.1016/j.nurx.2006.01.009. PMID: 16554257; PMCID: PMC3593444.
- Rosebush PI, Mazurek MF. Neurologic side effects in neuroleptic-naive patients treated with haloperidol or risperidone. Neurology. 1999 Mar 10;52(4):782-5. doi: 10.1212/wnl.52.4.782. PMID: 10078728.
- Silva RR, Muñoz DM, Daniel W, Barickman J, Friedhoff AJ. Causes of haloperidol discontinuation in patients with Tourette's disorder: management and alternatives. J Clin Psychiatry. 1996 Mar;57(3):129-35. PMID: 8617698.
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006 Jun 27;66(12):1817-23. doi: 10.1212/01.wnl.0000219679.95223.4c. PMID: 16801643.
- Purves D, Augustine GJ, Fitzpatrick D. Neuroscience. 2nd edition. Chapter 19. Modulation of Movement by the Cerebellum. Sunderland (MA): Sinauer Associates. 2001.
- Holmes G. The cerebellum of man. Brain. 1939; 62: 1-30.
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6(3):202-13. doi: 10.1080/14734220701266742. PMID: 17786816.
- Bracke-Tolkmitt R, Linden A, Canavan AGM, Rockstroh B, Scholz E, Wessel K, Diener HC. The cerebellum contributes to mental skills. Behav. Neurosci. 1989; 103(2): 442-446.
- Stephen CD, Brizzi KT, Bouffard MA, Gomery P, Sullivan SL, Mello J, MacLean J, Schmahmann JD. The Comprehensive Management of Cerebellar Ataxia in Adults. Curr Treat Options Neurol. 2019 Feb 21;21(3):9. doi: 10.1007/s11940-019-0549-2. PMID: 30788613.
- Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22-60. doi: 10.1159/000324065. Epub 2011 Apr 19. PMID: 21508625; PMCID: PMC4549070.
- Robins LN, Helzer JE, Davis DH. Narcotic use in southeast Asia and afterward. An interview study of 898 Vietnam returnees. Arch Gen Psychiatry. 1975 Aug;32(8):955-61. doi: 10.1001/archpsyc.1975.01760260019001. PMID: 1156114.
- Cavazos JE, Sanchez R. Pathophysiology of seizures and epilepsy. In Epilepsy: Scientific Foundations of Clinical Practice, Jong M. Rho, Raman Sankar, Jose E. Cavazos eds. 2005; 5-21.
- Sendrowski K, Sobaniec W. Hippocampus, hippocampal sclerosis and epilepsy. Pharmacol Rep. 2013;65(3):555-65. doi: 10.1016/s1734-1140(13)71033-8. PMID: 23950578.
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004 Apr;18(2):284-95. doi: 10.1037/0894-4105.18.2.284. PMID: 15099151.
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998 Sep;86(2):353-87. doi: 10.1016/s0306-4522(98)00004-9. PMID: 9881853.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
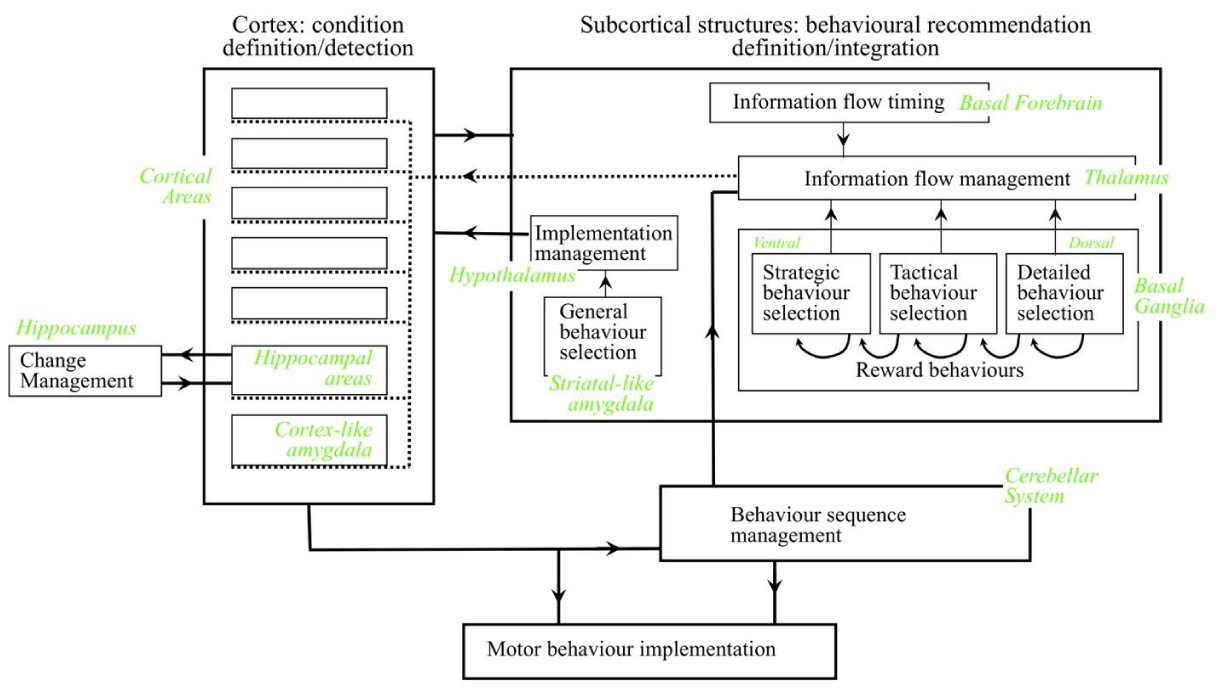
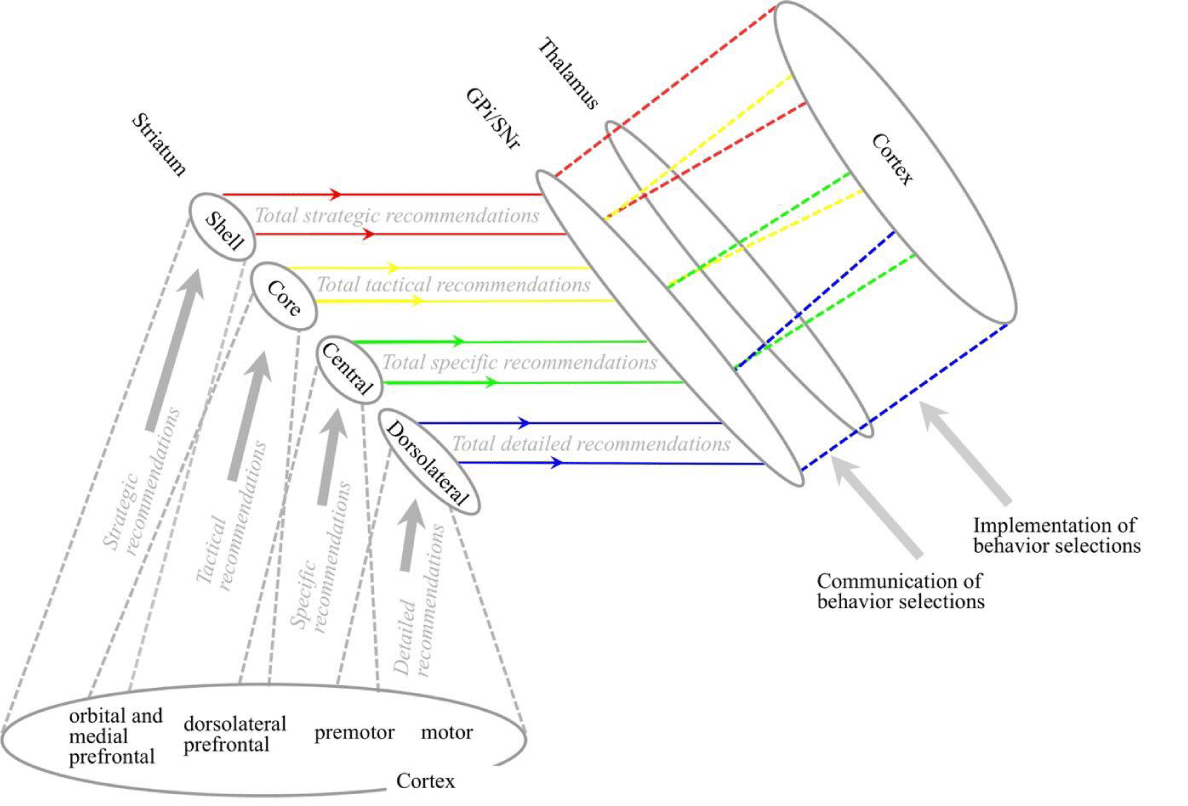
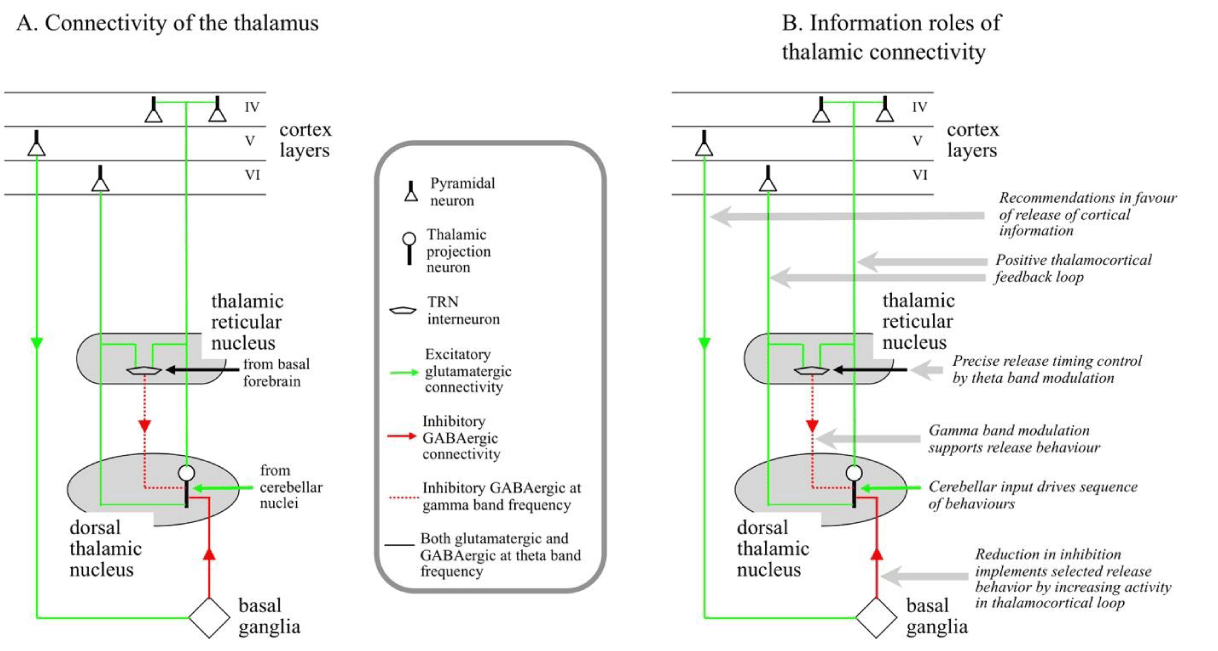
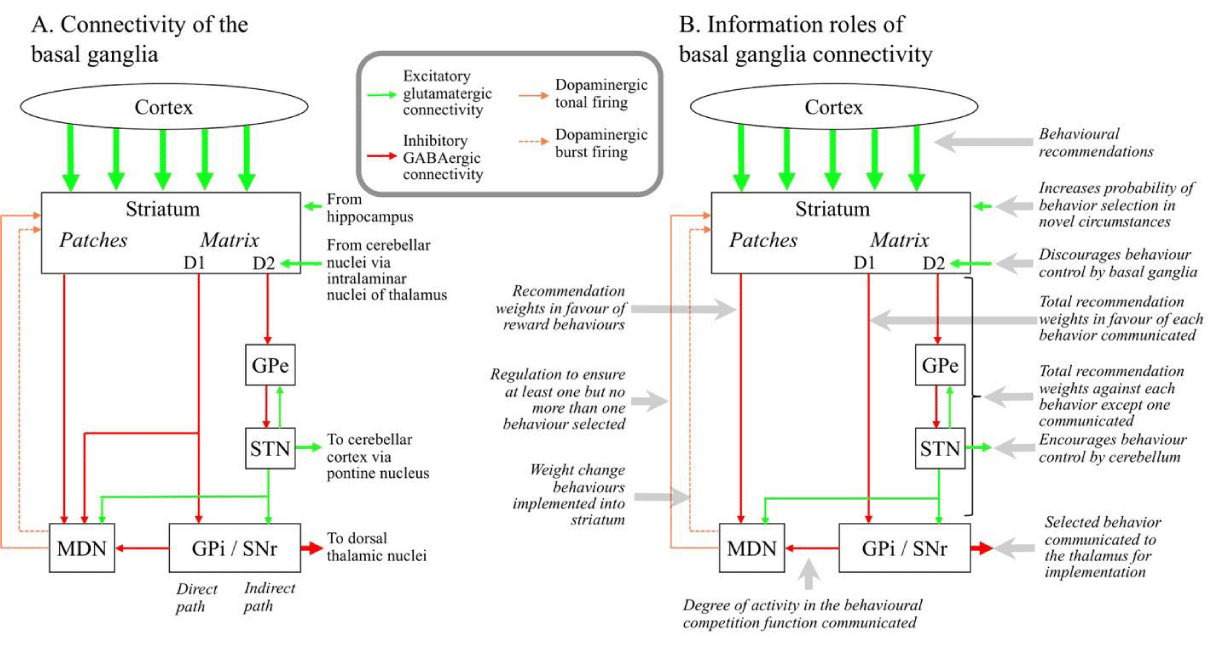
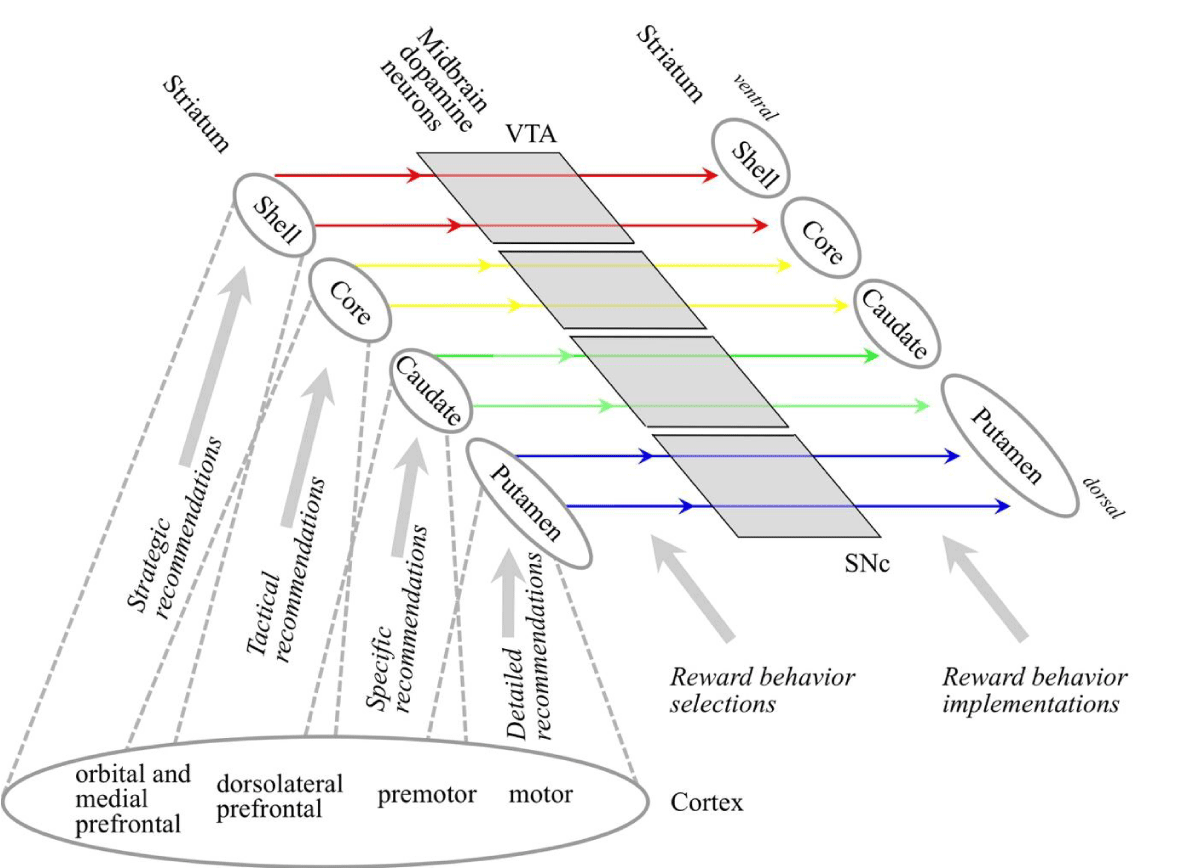
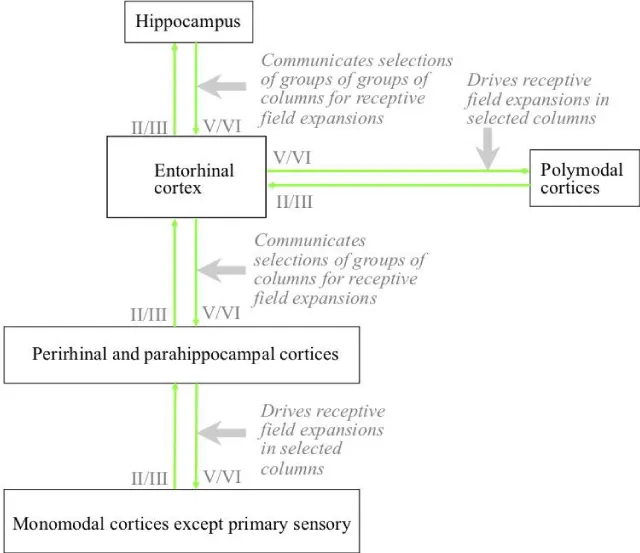
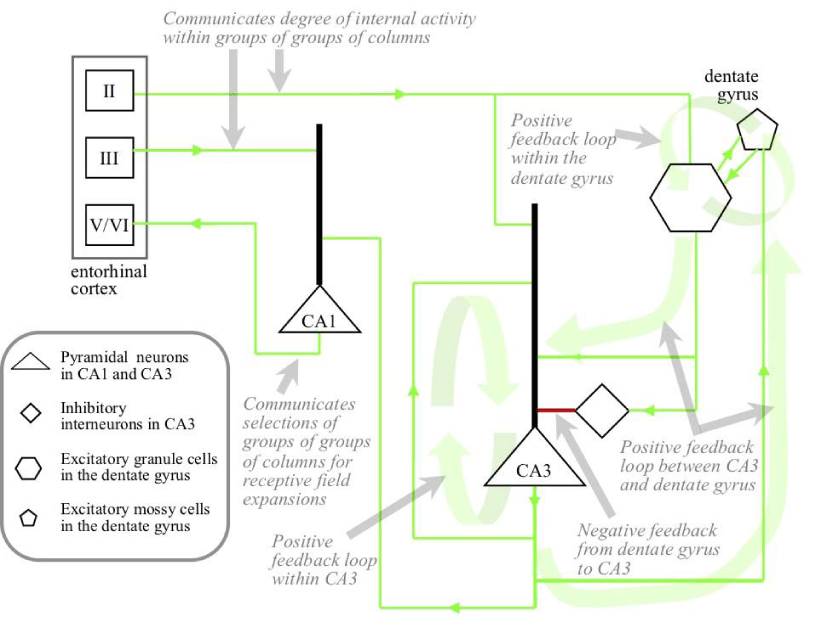
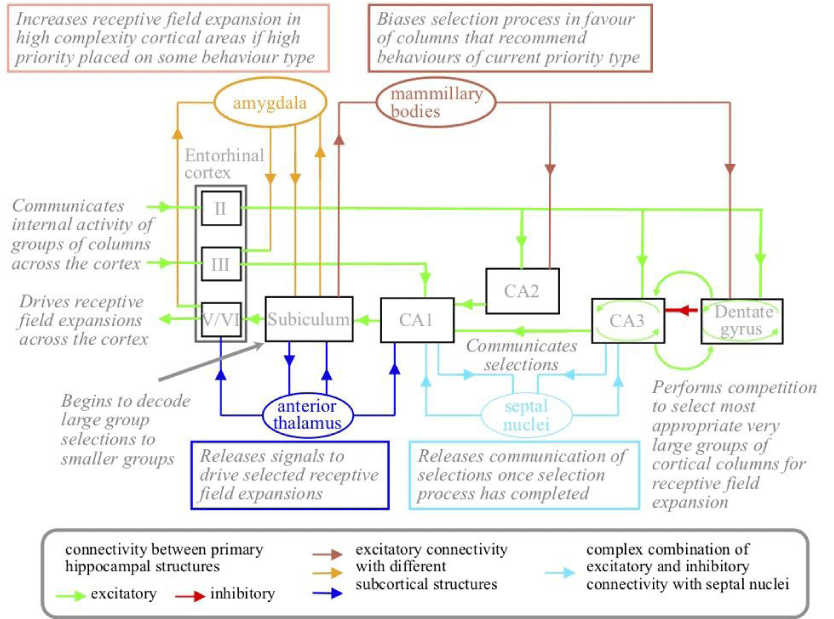
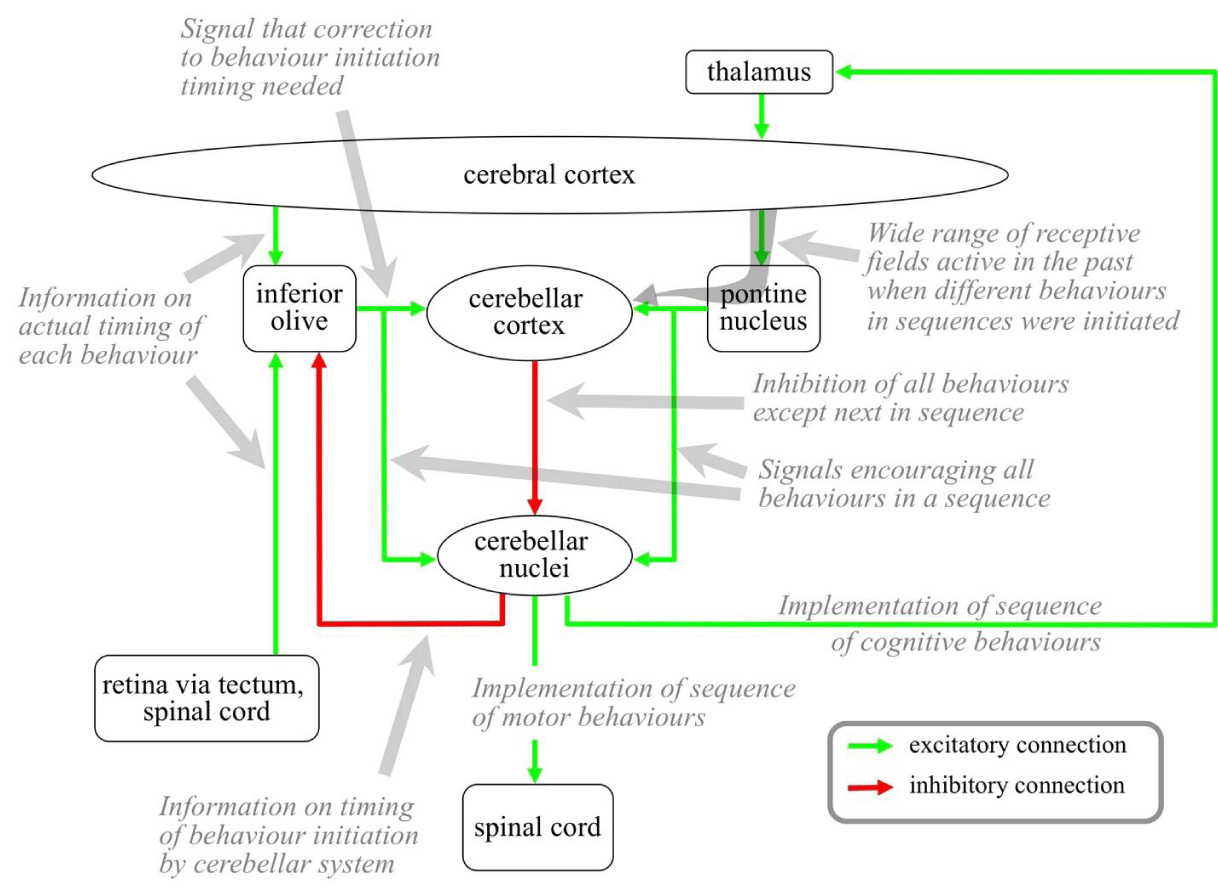
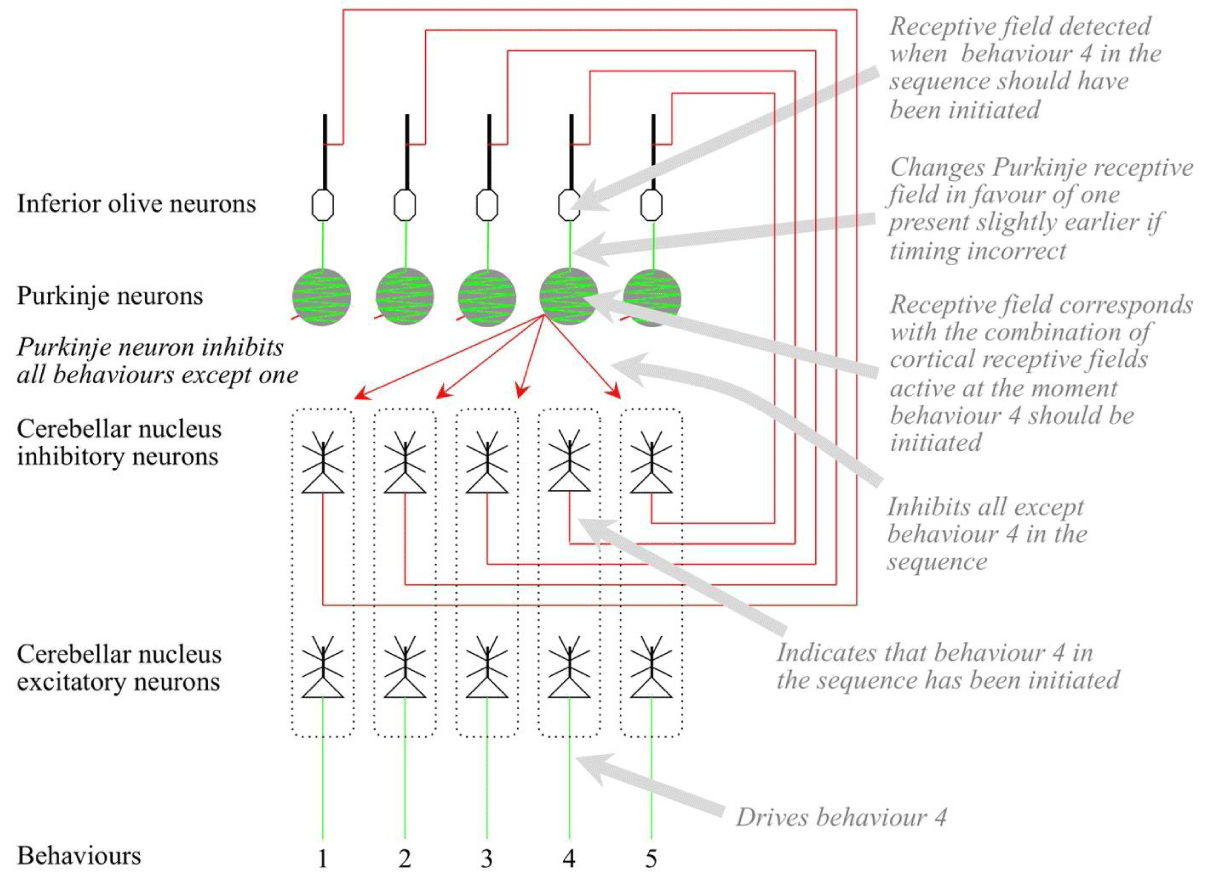
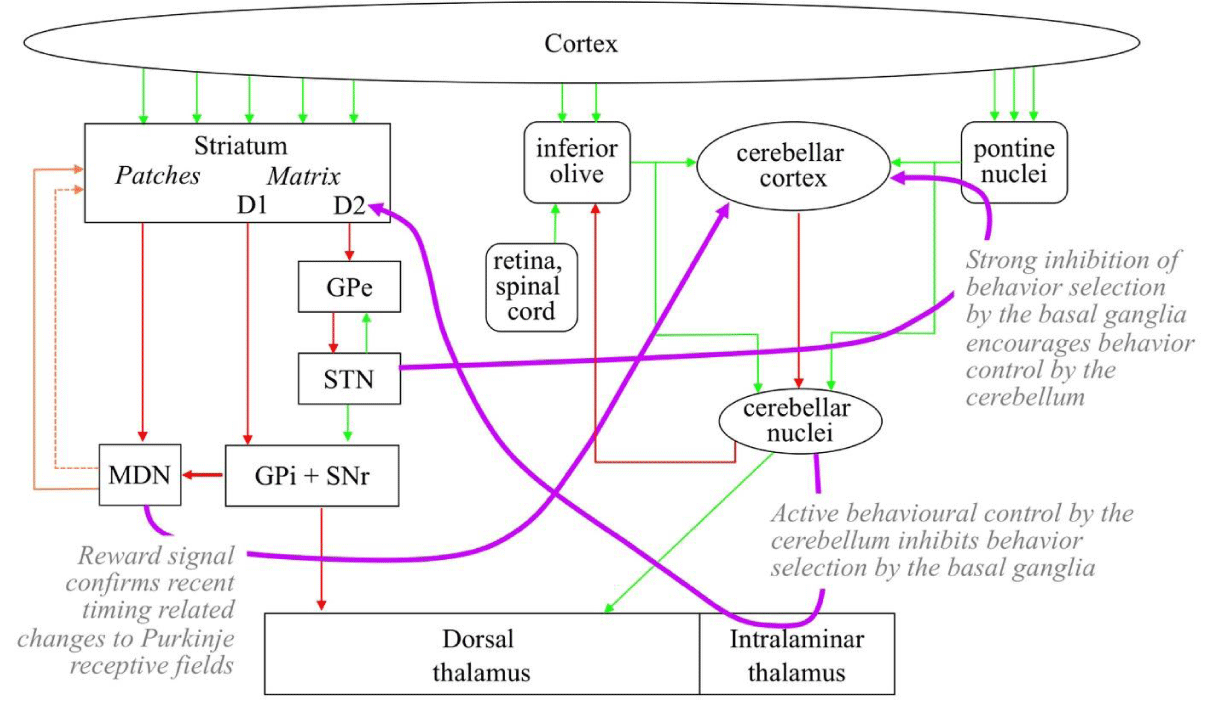


 Save to Mendeley
Save to Mendeley
