Annals of Alzheimer's and Dementia Care
Can Exposure to Volatile Anesthetics be a Tipping Point for AD Susceptible Populations?
Ingrid R Niesman*
Cite this as
Niesman IR (2017) Can Exposure to Volatile Anesthetics Be a Tipping Point for AD Susceptible Populations?. Ann Alzheimers Dement Care 2(1): 007-012. DOI: 10.17352/aadc.000002The relationship between surgery induced Postoperative Cognitive Dysfunction (POCD) and the development of Alzheimer’s disease (AD) later has been a debatable question. Volatile anesthetics represent a potential environmental factor that can change the CNS both acutely and long-term. By interacting with membrane cholesterol to alter signaling in neurons or alter the normally quiescent microglial phenotype, volatile anesthetics are implicated in the development of POCD. The tipping point for triggering AD cyclic pathology may rest with individual AD genetic risk factors combined with the known molecular consequences of anesthetic exposure. This review covers genome wide association studies (GWAS) identified AD risk factors, actions of volatile anesthetics in the development of AD phenotypes and presents some newly discovered pre or post-anesthetic POCD attenuating therapies.
Introduction
As a consequence of modern medicine and healthier living, our population is aging beyond our current understanding of geriatric physiology. One inevitable fact society must face is the growing number of cases of age-related cognitive decline and neurodegenerative diseases. The costs of these conditions in terms of monetary value and in terms of human quality of life are unquantifiable. We have identified numerous genetic and lifestyle risk factors for development of Alzheimer’s disease (AD), yet penetrance across populations is sporadic [1,2]. Thus, the actual mechanistic triggers or tipping points to initial the pathological cascades leading to AD or other forms of cognitive decline have remained elusive.
One of the most prominent risk factors for the development of late onset Alzheimer’s disease (LOAD) is the presence of heterozygous or homozygous ApoE4 alleles. ApoE and cholesterol have a complex inter-relationship in CNS. Astrocytes are the major producers of cholesterol and neurons are the major consumers, using the cholesterol transported by ApoE particles to build their elegant membrane structures and maintain the synaptic superhighway of information. Therefore, dysfunction in transport, uptake or trafficking of cholesterol may represent major contributing factors to the development of AD [3-5]. Along those lines of reasoning, other factors which potentially disrupt cholesterol biogenesis, synthesis and mechanisms of action, could exacerbate already damaged cells, leading to clinical pathology.
Volatile general anesthetics (vGA) interact directly with cholesterol in the plasma membrane, through an undefined mechanism, and alter signal transduction cascades [6]. These effects can be considered acute or chronic [7] depending on the precise protein or receptor affected. However, the actual chemical structure of individual anesthetics may dictate these interactions. Isoflurane (ISO) and sevoflurane (SEVO) have been shown to have opposing effects compared to desflurane (DES), due to differences in the degradative byproduct trifluoroacetic acid or the shorter action time [8,9].
Postoperative Cognitive Dysfunction (POCD) is a well described clinical syndrome found in elderly patients following surgery, with duration and symptomology widely varying and found to be associated with use of inhaled anesthetics. Any combination of AD risk factors and common surgical procedures may be considered a triggering event in the long pathological decline leading to full-blown AD disease. This review will discuss: a) the implications of vGA exposure in the development of cognitive impairment, b) theories supporting vGA as an initiating trigger of AD pathology and c) identify some potential therapeutic targets to attenuate the consequences.
Genetic risk factors and volatile anesthetic interactions
The Alzheimer’s Association (Alz.org) currently estimates around 5.3 million AD patients in the US alone and the number will escalate as the percentage of people >65 years increases in the population. Bioinformatics, combined with robust genomic analyses, have identified new cohorts of risk factors associated with development of sporadic Alzheimer’s disease (SAD) or LOAD. What we currently lack is an understanding of how the daily human experience and the molecular mechanisms of risk factors genes can synergize, leading to AD clinical manifestations. One significant environmental factor which has the potential to alter physiology both transiently and chronically, is the use of volatile anesthetics during surgical procedures [10]. Anesthetics such as ISO are commonly administered to all age populations and have been linked to early childhood cognitive issues [11] and elderly POCD [8]. Older adults represent a large percentage of surgical patients, with cardiovascular surgery and joint replacements common procedures requiring extensive use of anesthetics.
Physiological interactions at a molecular level between variant risk gene proteins and volatile anesthetics may likely determine the initiation of dementia and classical AD pathologies in any number of individuals. Familial Alzheimer’s disease (FAD) has a clear genetic component. Mutations and duplications of human amyloid precursor protein (APP) and the presenilins 1 and 2 (PSEN1; PSEN2) have been identified in large family cohorts of patients [12], but, these cases represent only a minor fraction of current clinical presentation. The etiology of the remaining cases is still an open debate. Newly identified AD risk variants, typically single nucleotide polymorphisms – SNPs - form interesting cluster patterns, ranging from cholesterol biology, intracellular trafficking to neuro inflammation [1,2,5,13-16]. Table 1 lists current information on genes with risk identified SNPs, cell type most affected and the cellular function if defined.
Precisely how these variant proteins can alter normal CNS homeostasis and result in AD related cognitive decline or how individuals with these genetic alleles avoid AD pathology are key questions. Until we have a significant pool of fully sequenced humans and a lifetime to study their particular nurturing and environmental interactions, the latter will remain unanswered and unobtainable. However, we have begun to unravel connections between AD risk factors and anesthetic exposure [10]. POCD has been clinically recognized and has clearly defined operational criteria [17]. How, when and where the transition or switch from simple POCD to the long pathway of synapse loss, neuronal damage and neuroinflammation occurs has been confounded by the use of transgenic AD mouse models, non-human CNS cell cultures and a lack of definitive long term human clinical studies.
Known volatile anesthetic effects
Volatile anesthetics interact directly with the lipid bilayer, modifying the local lipid and protein environment. Three theories have been proposed for the interactions of lipids and volatile anaesthetics: 1) direct binding to membrane proteins modifying their conformation and signaling activity; 2) binding to and weakening cholesterol and phospholipid associations which alters plasma membrane localized ion channel activity; and 3) binding specifically to lipid raft domains, modifying their lateral organization, interfering with many required protein-protein interaction in signal transduction pathways [18].
As the chemical structures of this class of anesthetics are diverse, the interactions with lipids and proteins are likely to differ and have differential effects. ISO has a weakening effect on cholesterol-phospholipid interaction in lipid raft domains, suggesting that ISO acts as a “replacement” or substitute for cholesterol [19,20]. Propofol (PPF), on the other hand, can interact with raft or non-raft domains and when PPF induced metabolic changes, after exposure, were compared to SEVO post-exposure, distinct differences in fatty acid or glucose oxidation were seen. SEVO increases fatty acid transport and PPF increases pyruvate dehydrogenase activity without affecting GLUT4 [20].
Within the CNS, these volatile anesthetics can interact differentially within the membrane environment based on phospholipid composition and differentially with local areas of membrane specialization, such as the pre or postsynaptic zones or lipid rafts. vGA effects may also be temporally different throughout lifespans. We are now realizing the profound phenotypic differences between young glia and old glia in terms of priming, cytoskeletal integrity and mitochondrial function. The same can be said for neuronal phenotypes; young cells are highly plastic, while older neurons have a myriad of dysfunctional cell operations, such as autophagy [21].
How volatile general anesthetics (vGA) can induce AD pathology
The hallmark pathology of AD has long been considered extracellular deposition of toxic Aβ – amyloid plaques - and/or the intracellular accumulation of oligomerized tau protein, known as neurofibrillary tangles (NFT), but this concept is currently challenged [22]. Still, the controversy rages on and recent data clearly suggests that these pathologies are probably symptomatic not causative [23]. vGAs have the capacity to increase Aβ processing [10,24,25]. Importantly, 2% ISO treatment for six hours drives β−secretase activity over α−secretase activity, increasing the Aβ1-42 toxic cleavage product, leading to aggregation over short time points [24,26]. When these cell culture studies are repeated in transgenic AD mouse models however, inconsistent data is found across compounds. In their 2011 review, Papon et al, conclude that although it appears little differences in APP processing occurs in normal mice following vGA exposures, transgenic mice with known increased Aβ plaque formation are at increased risk for increased aberrant APP processing following exposures [27].
Tau protein is a small molecular weight microtubule-associated protein. When bound to polymerized microtubules in neuronal axons, tau acts as stabilizing struts to maintain the shape and flexibility of long axonal processes. When phosphorylated by GSK-β3, a major effector kinase in this pathway, tau falls off the microtubule and depolymerization can begin. Things go awry when this phosphorylation process become unregulated or the known phosphatase PP2A loses functionality - reviewed by [28]. Tau is continually phosphorylated on multiple serine and threonine residues – hyperphosphorylation - an initiating step in the pre-tangle stages of tau aggregation [29,30]. vGA can accelerate this process by increasing pS and pThr sites directly [31] or through inhibition of PP2A [27]. A principle side effect of vGA exposure during surgical procedures is significant drop in body temperature, hypothermia. The enzymatic activity of PPA2A is directly tied to temperature, thus the lowered thermostat has been found to inhibit the dephosphorylation of tau by indirect means, abnormally increasing hyperphosphorylated tau [27,32,33]. SEVO induces increased p-tau/t-tau ratios via inhibition of mammalian target of rapamycin (mTOR); a pathway mediated by GSK-β3, indicating vGAs can directly influence intracellular kinase activities [34,35].
However, since both amyloid plaques and NFTs may take years to manifest and POCD is a more acute ramification of vGA exposure, other mechanisms are most likely in play. Zhang et al, have made a strong case that induction of neuroinflammation by vGA in older patients is the primary factor in the development of POCD [35]. Exposure to ISO or PPF in elderly rats increased IL-1β and increased expression of the microglial biomarker, Iba-1. Eight days post-surgery, the rats also displayed significantly longer target whole identification in a Barnes Maze learning and memory task. In addition, the rats showed reduced levels of freezing in fear conditioning behavioral tests. A second study directly compares adult and aged mouse cytokine expression and behavior three days post ISO exposure and finds elevated expression of canonical neuroinflammatory markers, such as IL-1β, TNF-α, IFN-γ, in the aged mice, along with impaired spatial learning memory [36]. Proposed models of AD pathology implicate neuroinflammation as a precipitating event in the development of disease [37,38], so these data represent a perplexing conundrum to clinicians. Do we risk exposing elderly patients to potentially harmful compounds and forgo life-saving care or do we recognize this as a significant clinical issue and begin to unravel the molecular causes to find a solution?
The search for an intervention when a mechanism is not yet fully elucidated: POCD to AD
Armed with the idea that neuroinflammation may be the underlying condition fueling POCD and ultimately AD, several groups have begun exploring novel anesthetic pre- or post-treatments to reduce the activation of microglia in the hippocampus and other brain regions, reviewed by [39]. Berberine is a natural isoquinoline alkaloid, with known anti-inflammatory properties, acting through suppression of the TLR-4/NF-κβ signaling pathway. It can cross the BBB but has pleiotropic effects and very low bioavailability. A recent 2016 study has found that 10mg/kg intraperitoneal (IP) injections of berberine following 1.5% ISO exposure in a mouse model during laproscopic surgery, was enough to reduce expression of canonical inflammatory markers and prevent POCD in a accepted clinical animal model of POCD [40]. The antibiotic minocycline has become a CNS anti-inflammatory drug of choice with the ability to cross the BBB and to moderate microglial phenotypes. When mice were pre-treated with 45 mg/kg IP minocycline 12 hours prior to 1.5 - 2% ISO exposure, POCD associated behavioural changes and markers of neuroinflammation were reduced. More importantly, the neuroprotective cytokines, IL-4 and IL-10 were concomitantly upregulated [41].
Two other compounds are also being evaluated, which have indirect anti-inflammatory properties. The α2-adrenergic receptor agonist, dexmedetomidine, has CNS protective effects. When administered 30 minutes prior to vGA exposure at 15-25µg/kg IP, activation of the α2-adrenergic receptor significantly reduced the expression of neuroinflammatory markers [42]. However, another study from 2014 demonstrated non-hypothermic dependent hyperphosphorylation of tau when dexmedetomidine was used as a standalone GA [43], making this compound less attractive as an interventional therapy. By an intriguing and novel mechanism, deferoxamine shows protection against POCD in aged rats, by reducing the activation of microglia following vGA exposure. Microglial activation is preceded by a burst in oxidative phosphorylation, mediated by iron containing mitochondrial proteins. Pan, et al, theorized chelation of iron for six days prior to surgery could attenuate POCD symptoms, by preventing the metabolic burst required for full microglial activation [44]. They found that they were able to stave off microglia activation by oxidative stress mechanisms, using deferoxamine, and were also able to prevent the associated neurotoxicity and cognitive deficits.
Two studies, published in early 2017, have intriguing data on other possible selective targets and pathways to mitigate POCD. The first study attenuates POCD associated neurodegeneration in aged mice with PPARγ activation using the agonist pioglitazone prior to ISO exposure. Antagonizing the receptor reverses the positive benefits of pre-treatment [45]. The already approved pioglitazone treatment for diabetes lowers blood sugar levels but has potential cardiac risks with long-term treatment. However, the suggestion that short term or acute administration prior to surgery can provide behavioural benefits makes this pathway an attractive target and well worth investigating in clinical trials. Since vGA exposure – SEVO in particular – increases p-tau/t-tau ratios and inhibits the autophagy pathway through mTOR [34,35], a compound that targets both p-tau generation and moderates autophagy pathways is another method worth investigating. The nutriceutical selenomethionine (Se-Met) has shown efficacy in the retention of cognitive function in mouse transgenic AD models [46]. This compound reduces p-tau by modulation of the key GSK-β3/PP2A pathway and concomitantly activates the AMPK-mTOR axis to enhance autophagic clearance. Animals were treated for 3-month, not short term, and the effects were modest but as proof of concept studies, they establish the basis to screen for other similar compounds with faster kinetics and pharmacodynamics.
Volatile anesthetics and the “Two-Hit” concept of AD
Although many studies have established the vGAs have the ability to mimic or induce AD phenotypes, the definitive connection between development of POCD and the long road to AD dementia is unclear. Current thinking about the trek down the cyclic path towards AD is muddied by the failures of promising anti-Aβ therapies (solanezumab – Eli Lilly, 2016). Newly emerging hypotheses revolve around the concept of multiple small causative risk factors combining together to create a favorable environment for loss of synapses and neuronal death to occur. The Two-Hit hypothesis was initially proposed in 2004 by Zhu, et al, [47] and refined by Zlokovic [48] in 2011. The relevance of this concept has been greatly strengthened with genome wide association studies (GWAS) variant identifications [1,2]. In theory, patients can have heterozygous variant risk genes across specific CNS cell types. In a homeostatic situation, these individual risk factors play a margin role in brain dysfunction. However, if contributing second factors, like a concussion (TBI), aberrant glucose utilization, metabolic cholesterol dysfunction, or exposure to vGA, are added, the balance can shift to pathogenic pathways.
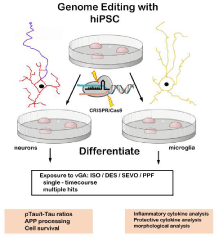
Whether exposure to vGA is an accelerating factor in people predisposed to AD by eliciting POCD through neuroinflammatory routes or by directly inducing initial Aβ or p-tau mechanisms needs more experimental data. One idea worth exploring is the use of CRISPR/Cas9 genome-edited human induced pluripotent (hiPSC) stem cells to directly model the effects of vGA on wild-type verses genetic variants of each CNS cell type [49-52]. (Figure 1) Use of relevant human cells instead of transgenic animal models provides a more accurate in vivo picture and breaking down the potential affects, based on cell type, provides a solid foundation to view the combined phenotypes. By this decomposition methodology, we can gain valuable insights into acute and chronic effects of each vGA and hopefully use that knowledge to design appropriate anesthetic protocols for each patient based on individual genetic make-ups.
Conclusion
Considering our reliance on volatile general anesthetics in modern medicine, it is surprising how little we actually understand about the immediate and long-term consequences of exposures. Clearly these complex compounds interact on a molecular level with cholesterol in cell membranes and with many genetic AD risk factor variants clustered in cholesterol metabolic pathways, much more precise information about is needed to understand the vGA effects on variant human neurons. The same can be reiterated about microglial function and phenotype. Newly discovered variants specific for these cells are clustered around phagocytosis and markers of activation. Whether vGA can destabilize already primed variant microglia, to elicit enhanced neuroinflammation, is a key question that needs further study.
- Chouraki V, Seshadri, S (2014) Genetics of Alzheimer's disease. Adv Genet 87: 245-294. Link: https://goo.gl/q6W2cx
- Marei HE, Althani A, Suhonen J, Zowalaty El ME, Albanna MA, et al. (2016) Common and Rare Genetic Variants Associated With Alzheimer's Disease. J Cell Physiol 231: 1432-1437. Link: https://goo.gl/ShTw33
- Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9: 106-118. Link: https://goo.gl/VQzKwY
- Holtzman DM, Herz J, Bu G (2012) Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2: a006312. Link: https://goo.gl/Wx24kt
- Dong HK, Gim JA, Yeo SH, Kim HS (2017) Integrated late onset Alzheimer's disease (LOAD) susceptibility genes: Cholesterol metabolism and trafficking perspectives. Gene 597: 10-16. Link: https://goo.gl/6Pz2Ue
- Egawa J, Pearn ML, Lemkuil BP, Patel PM, Head BP (2016) Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function. J Physiol 594: 4565-4579. Link: https://goo.gl/Ejq230
- Qiu L, Zhu C, Bodogan T, Gomez-Galan M, Zhang Y, et al. (2016) Acute and Long-Term Effects of Brief Sevoflurane Anesthesia During the Early Postnatal Period in Rats. Toxicol Sci 149: 121-133. Link: https://goo.gl/i4vNIH
- Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, et al. (2012) The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg 114: 410-415. Link: https://goo.gl/4dnN2o
- Vlisides P, Xie Z (2012) Neurotoxicity of general anesthetics: an update. Curr Pharm Des 18: 6232-6240. Link: https://goo.gl/SmVJkE
- Bittner EA, Yue Y, Xie Z (2011) Brief review: anesthetic neurotoxicity in the elderly, cognitive dysfunction and Alzheimer's disease. Can J Anaesth 58: 216-223. Link: https://goo.gl/bZH5BQ
- McCann ME, Soriano SG (2009) Is anesthesia bad for the newborn brain? Anesthesiol Clin 27: 269-284. Link: https://goo.gl/sSFyLy
- Rosenberg RN, Lambracht-Washington D, Yu G, Xia W (2016) Genomics of Alzheimer Disease: A Review. JAMA Neurol 73: 867-874. Link: https://goo.gl/cd6fVq
- Kamboh MI, Demirci FY, Wang X, Minster RL, Carrasquillo MM, et al. (2012) Genome-wide association study of Alzheimer's disease. Transl Psychiatry 2: e117. Link: https://goo.gl/ExjhzC
- Lambert JC, Ibrahim VCA, Harold D, Naj AC, Sims R, et al. (2013) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 45: 1452-1458. Link: https://goo.gl/Ns4xHz
- Karch CM, Goate AM (2015) Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77: 43-51. Link: https://goo.gl/71WT0l
- Villegas LC, Phillips A, Garcia RP, Hardy J, Pocock JM (2016) Microglial genes regulating neuroinflammation in the progression of Alzheimer's disease. Curr Opin Neurobiol 36: 74-81. Link: https://goo.gl/kLJpex
- Tsai TL, Sands LP, Leung JM (2010) An Update on Postoperative Cognitive Dysfunction. Adv Anesth 28: 269-284. Link: https://goo.gl/cfCBmm
- Turkyilmaz S, Almeida PF, Regen SL (2011) Effects of isoflurane, halothane, and chloroform on the interactions and lateral organization of lipids in the liquid-ordered phase. Langmuir 27: 14380-14385. Link: https://goo.gl/hnHe09
- Bandeiras C, Serro AP, Luzyanin K, Fernandes A, Saramago B (2013) Anesthetics interacting with lipid rafts. Eur J Pharm Sci 48: 153-165. Link: https://goo.gl/KRCL7i
- Wang L, Ko KW, Lucchinetti E, Zhang L, Troxler H, et al. (2010) Metabolic profiling of hearts exposed to sevoflurane and propofol reveals distinct regulation of fatty acid and glucose oxidation: CD36 and pyruvate dehydrogenase as key regulators in anesthetic-induced fuel shift. Anesthesiology 113: 541-551. Link: https://goo.gl/0YSDa1
- Wong YC, Holzbaur EL (2015) Autophagosome dynamics in neurodegeneration at a glance. J Cell Sci 128: 1259-1267. Link: https://goo.gl/tNatsK
- Herrup K (2015) The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 18: 794-799. Link: https://goo.gl/tf5IdZ
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, et al. (2011) Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 69: 181-192. Link: https://goo.gl/ucM5lI
- Xie Z, Tanzi RE (2006) Alzheimer's disease and post-operative cognitive dysfunction. Exp Gerontol 41: 346-359. Link: https://goo.gl/OB7LT5
- Bianchi SL, Tran T, Liu C, Lin S, Li Y, et al. (2008) Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging 29: 1002-1010. Link: https://goo.gl/4su0L3
- Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, et al. (2006) The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 104: 988-994. Link: https://goo.gl/bQlusm
- Papon MA, Whittington RA, El-Khoury NB, Planel E (2011) Alzheimer's disease and anesthesia. Front Neurosci 4: 272. Link: https://goo.gl/GSdoYe
- Hoffman A, Taleski G, Sontag E (2017) The protein serine/threonine phosphatases PP2A, PP1 and calcineurin: A triple threat in the regulation of the neuronal cytoskeleton. Mol Cell Neurosci. Link: https://goo.gl/8kNDK0
- Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, et al. (2004) Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol 63: 287-301. Link: https://goo.gl/lfSzck
- Sontag E, Nunbhakdi CV, Lee G, Bloom GS, Mumby MC (1996) Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17: 1201-1207. Link: https://goo.gl/XGCtZf
- Tao G, Zhang J, Zhang L, Dong Y, Yu B, et al. (2014) Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology 121: 510-527. Link: https://goo.gl/udB08V
- Tan W, Cao X, Wang J, Lv H, Wu B, et al. (2010) Tau hyperphosphorylation is associated with memory impairment after exposure to 1.5% isoflurane without temperature maintenance in rats. Eur J Anaesthesiol 27: 835-841. Link: https://goo.gl/F8uBRC
- Whittington RA, Bretteville A, Dickler MF, Planel E (2013) Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry 47: 147-155. Link: https://goo.gl/hvZbqA
- Liu Y, Ma L, Jiao L, Gao M, Guo W, et al. (2015) Mammalian target of rapamycin/p70 ribosomal S6 protein kinase signaling is altered by sevoflurane and/or surgery in aged rats. Mol Med Rep 12: 8253-8260. Link: https://goo.gl/du2TrI
- Zhang J, Tan H, Jiang W, Zuo Z (2015) The choice of general anesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in elderly rats. J Neuroimmune Pharmacol 10: 179-189. Link: https://goo.gl/KjRjKQ
- Wang HL, Ma RH, Fang H, Xue ZG, Liao QW (2015) Impaired Spatial Learning Memory after Isoflurane Anesthesia or Appendectomy in Aged Mice is Associated with Microglia Activation. J Cell Death 8: 9-19. Link: https://goo.gl/a0l2qg
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383-421. Link: https://goo.gl/Da6LGT
- Hu Z, Ou Y, Duan K, Jiang X (2010) Inflammation: a bridge between postoperative cognitive dysfunction and Alzheimer's disease. Med Hypotheses 74: 722-724. Link: https://goo.gl/f8Lyv0
- Kapila AK, Watts HR, Wang T, Ma D (2014) The impact of surgery and anesthesia on post-operative cognitive decline and Alzheimer's disease development: biomarkers and preventive strategies. J Alzheimers Dis 41: 1-13. Link: https://goo.gl/zf49k9
- Zhang Z, Li X, Li F, An L (2016) Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol 38: 426-433. Link: https://goo.gl/vTB89b
- Wang HL, Liu H, Xue ZG, Liao QW, Fang H (2016) Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med 20: 1632-1639. Link: https://goo.gl/XlOzMW
- Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang JM, et al. (2015) Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur J Pharmacol 746: 206-212. Link: https://goo.gl/JVeHEk
- Huang C, Ho YS, Ng OT, Irwin MG, Chang RC, et al. (2015) Dexmedetomidine directly increases tau phosphorylation. J Alzheimers Dis 44: 839-850. Link: https://goo.gl/55XooZ
- Pan K, Li X, Chen Y, Zhu D, Li Y, et al. (2016) Deferoxamine pre-treatment protects against postoperative cognitive dysfunction of aged rats by depressing microglial activation via ameliorating iron accumulation in hippocampus. Neuropharmacology 111: 180-194. Link: https://goo.gl/xpcyPT
- Zhang Z, Yuan H, Zhao H, Qi B, Li F, et al. (2017) PPARgamma activation ameliorates postoperative cognitive decline probably through suppressing hippocampal neuroinflammation in aged mice. Int Immunopharmacol 43: 53-61. Link: https://goo.gl/QSFrxH
- Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, et al. (2017) Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer's disease mouse model. J Neurosci Link: https://goo.gl/pwmSE3
- Zhu X, Raina AK, Perry G, Smith MA (2004) Alzheimer's disease: the Two-Hit hypothesis. Lancet Neurol 3: 219-226. Link: https://goo.gl/J9ZoRg
- Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12: 723-738. Link: https://goo.gl/a1WhC0
- Ito H, Uchida T, Makita K (2015) Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons. PLoS One 10: e0128445. Link: https://goo.gl/kaNWef
- Li S, Xue H, Long B, Sun L, Truong T, et al. (2015) Efficient generation of hiPSC neural lineage specific knockin reporters using the CRISPR/Cas9 and Cas9 double nickase system. J Vis Exp e52539. Link: https://goo.gl/SyHFMC
- Hockemeyer D, Jaenisch R (2016) Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell 18: 573-586. Link: https://goo.gl/L67Qm0
- Mungenast AE, Siegert S, Tsai LH (2016) Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci 73: 13-31. Link: https://goo.gl/VWYEyA

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
