Annals of Alzheimer's and Dementia Care
Microtubule-associated protein 1B rescues memory decline in Alzheimer’s disease model mice
Yoshimi Mitsuyama1, Tadashi Koide2, Tetsuo Kanno3, Shizuko Nagao4, Takayuki Manabe5-7 and Fuyuki Mitsuyama3,8*
2Fukuyu Medical Institute, Nagoya, Japan
3Department of Neurosurgery, Fujita Health University, Aichi, Japan
4Education and Research Center of Animal Models of Human Diseases, Fujita Health University, Aichi, Japan
5Division of Gene Expression Mechanism, Institute for Comprehensive Medical Science, Fujita Health University, Aichi, Japan
6Laboratory for Neuroanatomy and Neuropharmacology, Research Field of Professional Basic Medicine, Department of Nursing, Faculty of Nursing, Chukyogakuin University, Gifu, Japan
7Department of Child Development and Molecular Brain Science (Osaka University), United Graduate School of Child Development, Osaka University, Kanazawa University, Hamamatsu University School of Medicine, Chiba University, University of Fukui, Osaka, Japan
8Department of Internal Medicine, Tenjyu Hospital, Nagoya, Japan
Cite this as
Mitsuyama Y, Koide T, Kanno T, Nagao S, Mitsuyama F, et al. (2018) Microtubule-associated protein 1B rescues memory decline in Alzheimer’s disease model mice. Ann Alzheimers Dement Care 2(1): 001-006. DOI: 10.17352/aadc.000005Background: The classic pathologies seen in Alzheimer’s disease (AD) are amyloid plaques and neurofibrillary tangles, but synapse and spine loss have been recognised as new pathologies. Microtubules are thought to be less plentiful in spines, so it has been thought that spine shape change and molecular transportation in spines is performed mainly by actin. However, reports of the intraspinal invasion of microtubules, alternative mechanisms require investigation. Microtubule-associated protein 1B has microtubule conserving and polymerising effects and is overexpressed in Fragile X syndrome, in which spines are thin and elongated. Fragile X protein is an mRNA-binding protein and as mRNA is transported along microtubules as RNA granules by kinesine family, we suspected that Fragile X protein is conjugated with kinesin family tail and RNA granules. As a result, the mutation of this protein may cause impairment of mRNA transport to spines. This could result in low local protein synthesis in spines that may induce thin spines, and finally inducing MAP1B overexpression by a negative feedback mechanism. As a result, intraspinal microtubules may be elongated and spines may be elongated. It is speculated that the polymerisation of these intraspinal microtubules by MAP1B may restore spine integrity and rescue AD symptoms, however, this has not yet been proven.
Method: We injected a Map1b-lentivirus chimera to the hemi-hippocampus of AD-model mice. The spatial working memory was assessed by the Y-maze and compared with non-injected mice. The change in spines by MAP1B overexpression in cultured neurons was investigated.
Results: The overexpression of Map1b to the hemi-hippocampus of AD model mice rescues memory impairment. Spatial working memory assessed by the Y-maze in injected mice improved to almost normal levels within 2 days of the injection. The overexpression produced microtubule-dense remarkably enlarged spines in the cultured neurons. Map1b-lentivirus chimera injection also restored reduced postsynaptic densities in AD model mice, as assessed by protein immunoblots.
Conclusions: These results suggest that MAP1B-dependent intraspinal microtubules may enhance the structural integrity of spines, restoring spine shrinkage, improving the bidirectional transportation of memory-facilitating molecules, and rescuing memory impairment in AD model mice.
Abbreviations
AD: Alzheimer’s Disease; MAP1B: Microtubule-Associated Protein 1B; MAPs: Microtubule-Associated Proteins; APP: Amyloid Precursor Protein; PS1: Presenilin 1; MMRRC: Mutant Mouse Regional Resource Center; PME: PIPES, MgCl2, EGTA buffer; GFP: Green Fluorescent Protein; PSD 95-YFP: Postsynaptic Density 95-Yellow Fluorescent Protein; SEM: Standard Error of the Mean; DIV: DAY in vitro; PSD: Postsynaptic Density; HOMER1; Homer Scaffolding Protein 1;
Introduction
The classic pathologies seen in Alzheimer’s disease (AD) are amyloid plaques [1] and neurofibrillary tangles [2], but synapse and spine loss [3-5], have been recognised as new pathologies. The mechanism of new pathologies in AD requires investigation. It has been thought that few microtubules are present in dendritic spines, with many in dendritic shafts and axons [6]. We suspected that intraspinal microtubules may be very unstable, and would only be observed if we used a microtubule-preserving fixation technique. Using acute hippocampal slices, long term potentiation (LTP)-producing stimulation, microtubule-preserving fixation [7] and electron microscopy, we previously found and reported, for the first time, the existence of stimulation-dependent intraspinal microtubules, which conjugate dendritic shafts and postsynaptic densities after stimulation [8-11]. Three other labs repeated this finding [12-14]. These other labs mostly used live cultured neurons and microscopy to show the temporal entry of microtubules into spines.
Microtubule-associated proteins (MAPs) form regularly spaced projections from microtubules and consist of tau, MAP1A/MAP1B, and MAP2. MAPs have microtubule conserving and polymerising effects, and their phosphorylation causes depolymerisation of microtubules. MAP1B has been thought to reside mainly in axons, but has recently been reported to also be present in dendrites and spines [15].
Fragile X syndrome is a disorder often associated with intellectual deficit. In the neurons of Fragile X syndrome patients, dendritic spines are thin and elongated, and MAP1B is overexpressed [16]. Fragile X protein is an mRNA-binding protein [16], and as mRNA is transported along microtubules as RNA granules by kinesine family [17], we suspected that Fragile X protein is conjugated with kinesin family tail and RNA granules. As a result, the mutation of this protein may cause impairment of mRNA transport to spines. This could result in low local protein synthesis in spines that may induce thin spines, and finally inducing MAP1B overexpression by a negative feedback mechanism. As a result intraspinal microtubules may be elongated and spines may be elongated. We aimed to determine what controls intraspinal microtubules, with MAPs being the most promising contenders. We hypothesised that MAP1B overexpression in the hippocampus may rescue the symptoms of AD by restoring spine loss or shrinkage.
Materials and Methods
Animal preparation
All animal procedures followed the National Institutes of Health guidelines, and all procedures were performed in accordance with the guidelines of the Education and Research Centre of Animal Models for Human Diseases in Fujita Health University. The experimental protocols were approved by the Ethics Committee of the Fujita Health University. To ascertain whether MAP1B could be used as a therapeutic agent for AD, we used Tg6799 mice as a mouse model. This model has five mutations in the amyloid precursor protein (APP) and presenilin 1 (PS1) genes and a rapid appearance of symptoms [18].
Map1b-lentivirus chimera-injection
It has been reported that amyloid deposition starts very early (from 2 months) and that poor performance in the Y-maze test can be seen from 4–5 months of age in APP/PS1 double transgenic AD model mouse [18]. The transgenic AD mouse [18], purchased from MMRRC, were anesthetized with isoflurane (2-3 % for maintenance). These APP/PS1 double transgenic mice co-express five Familiar Alzheimer’s Disease mutations. Each animal’s head was restrained and 1 µl of Map1b-lentivirus chimera solution was stereotaxically microinjected into the right hippocampus using mice-head fixation and microinjection system (Narisige, Tokyo, Japan). The injections were administered 2 mm laterally from the bregma, 2 mm posteriorly from the bregma, and 1 mm from the brain surface.
Y-maze test
Memory impairment was assessed using the Y-maze test (Map1b-lentivirus chimera-injected Tg6799 mice, n = 9; control Tg6799 mice, n = 10). The Y-maze parameters are as follows: size of arms, 40 cm; height, 10 cm (Muromachi Machine, Tokyo, Japan). There was no trial run. The temperature was controlled at 21°C. When each mouse is put in the center of the Y-maze, it begins searching. Normal mice tend to search a new arm after searching one arm. The number of arms entered in 8 minutes is counted. The number of good sets, visiting the three different arms (for example ABC), is divided by the number of good + bad three sets (e.g. ACA) during the 8-minute session. This is then multiplied by 100 to give the alternation percentage. When the mouse did not perform the searching action, the data were excluded.
Primary culture and transfection
Twenty-three 18-day-old C57BL/6 normal pregnant mice (SLC, Hamamatsu, Japan) (wild type mice of the same strain as the AD model mouse) were used. The mice were sacrificed by cervical dislocation. The hippocampi were removed, dissected, and primary cultured on the bottom of 4-slide culture chambers (Corning Incorporated, New York, USA) as described elsewhere [19]. At DIV14, 2 µl of Map1b-lentivirus chimera solution was added to each chamber. At DIV18, the cells were fixed using a microtubule-preserving fixation system [7] as follows: 8% paraformaldehyde was dissolved by heating in PIPES, MgCl2, EGTA (PME) buffer consisting of 0.1 M PIPES (pH 6.9), 1 mM MgCl2, and 1 mM EGTA at 100°C. Glycerol (microtubule-stabilizer) was added to a final concentration of 30% of the paraformaldehyde solution. Finally, Paclitaxel (microtubule-stabilizer, 1 mg/100 ml; Wako Pure Chemical, Osaka, Japan) was added. The room temperature was maintained at 37°C to avoid cooling of the specimens. Sufficient fixative solution was maintained at 37°C in a water bath to also avoid cooling the specimens. Prefixative opening of the CO2 chamber door for cell observation was avoided, lest the shape and molecular distribution supported by the microtubules be lost. At DIV17, GFP or PSD95-YFP were transfected as described [20]. As transfection controls of GFP and PSD-95-YFP, we performed the same protocol with no cDNA, resulting almost no expression (data not shown). As transfection controls for the Map1b-lentivirus chimera, we used Map1b alone, and no enlarged spines were seen (data not shown).
Imaging
Fluorescence microscopy was performed using an A1 confocal microscope (Nikon, Tokyo, Japan) and a BX-51 polarising microscope (Olympus, Tokyo, Japan). After cells cultured on 4-slide culture chambers were separately transfected with GFP or PSD95-YFP, cells in every chamber were fixed and immunostained using an anti-tubulin antibody (mouse, DM1A, x100, Sigma, Tokyo, Japan) and anti-mouse Alexa 594 (x100, Thermo Fisher Scientific Inc., MA, USA) as described [21]. After the immunostaining, the side walls of 4-slide culture chambers were removed and cells were mounted using coverslips and ProLong Diamond (Thermo Fisher Scientific, MA). Imaging experiments using cultured neurons were repeated more than three times.
DNA construction
Mouse Map1b was cloned from a mouse genome library which was produced from polyA RNA and Map1b sequence was verified. Mouse Map1b was conjugated with the lentiviral vector using Lenti-X™ 293T Cell Line and Lenti-X HTX Packaging System (Takara Bio Inc, Shiga, Japan).
Immunoblot analysis
Four Tg6799 mice were anesthetized with isoflurane. The brains were removed and the hippocampi were dissected for biochemical analysis. For immunoblotting, each hippocampus was homogenised in the same volume of 4× Laemmli sample buffer (Bio-Rad Laboratories, Tokyo, Japan) containing protease inhibitor cocktail (Calbiochem, Darmstadt, Germany) in a 1 ml Eppendorf tube. A 10–20 µl aliquot of each sample was run on 12% mini-protean TGX gels (Bio-Rad Laboratories, Tokyo, Japan). After transfer to polyvinylidene difluoride membranes (Amersham Hybond P PVDF, GE Health Care Japan, Tokyo, Japan), the membranes were incubated in the following primary antibodies: HOMER1 rabbit polyclonal antibody (1:500; Proteintec Group Inc., Rosemont, IL, USA) and anti-tubulin (1:10,000; DM1A, Sigma, St. Louis, MO), which was used as an internal control. After that, the membranes were incubated with peroxidase-conjugated secondary antibodies (Amersham), and signals detected by Image Quant 400 (GE Health Care, Buckinghamshire, England). We tested several antibodies as markers of the postsynaptic density, before selecting HOMER1 rabbit polyclonal antibody for our investigations. Quantitative band patterns were obtained using ImageJ (NIH).
Data analysis
All data are expressed as means ± standard error of the mean (SEM). Statistical analyses for two-sample comparisons were performed by a two-tailed Student’s t-test or one-way analysis of variance following estimation of least significant differences.
Results
Effect of hemi-hippocampal MAP1B injection on memory impairment measured by Y-maze test in Tg6799 mice (AD model mice)
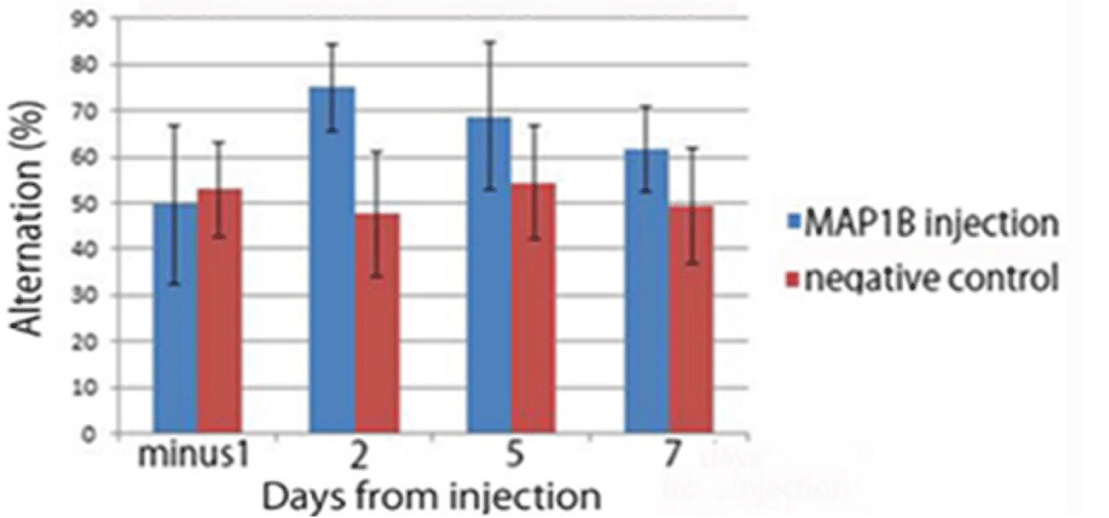
We injected a Map1b-lentivirus chimera to the hemi-hippocampus (right side) of nine Tg6799 mice at 101 days old. The spatial working memory of injected mice was assessed by spontaneous alternation in the Y-maze (n = 9) and compared with non-injected Tg6799 mice (n = 10). Memory deficits in the Tg6799 mice were improved by MAP1B overexpression. Both the injected and non-injected Tg6799 mice showed poorer alternation performance in the Y-maze the day before the injection (slightly above 50% chance levels). However, the MAP1B treated group showed a remarkable improvement in alternation performance in the Y-maze during the week following the injection (Figure 1). This improvement appears to reduce with time in the injected group.
The change in spines resulting from MAP1B overexpression in cultured neurons of wild type mice
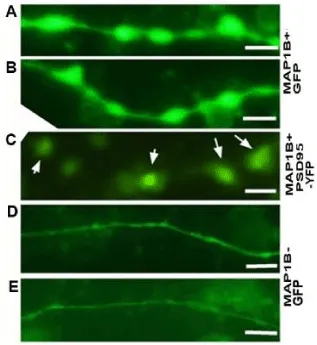
To clarify the mechanisms by which memory deficits are improved by MAP1B overexpression, we investigated the change in spines resulting from MAP1B overexpression in cultured neurons of wild type mice from the same strain as the model mice (C57/BL6). At DIV14, 2 µl of Map1b-lentivirus chimera solution was added to each cultured chamber (Figure 2A-C) and at DIV18, cells were fixed using a microtubule-preserving fixation method [7]. At DIV17, GFP (green fluorescent protein) (Figure 2A,B,D, and E) or PSD (postsynaptic density)-95 (representative protein of postsynaptic density which localizes to the top of the spines)-YFP (yellow fluorescent protein) (Figure 2C) was transfected into the cells. After 4 days of incubation with the Map1b-lentivirus chimera, remarkable protrusions of dendrites appeared (Figure 2A,B), compared with the negative controls, which had normal spines as delineated by GFP (Figure 2D,E). In cells co-transfected with PSD95-YFP and Map1b-lentivirus chimera, remarkable protrusions were slightly delineated by PSD-95-YFP. This indicates that Map1b is overexpressed in these cells. Furthermore, PSD-95-YFP high density spots were present in the protrusions (Figure 2C), suggesting that the protrusions of dendrites appearing following Map1b-lentivirus chimera administration may be enlarged spines.
The parts of the shaft microtubules participate in filling enlarged spines
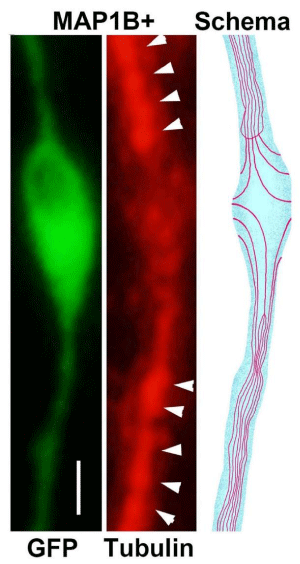
To investigate the relationship between microtubules and enlarged spines, cells co-transfected with GFP and a Map1b-lentivirus chimera were simultaneously stained with anti-tubulin antibody. The normal dendritic shaft was densely stained with anti-tubulin antibody. However, in the enlarged spines, dendritic shafts had lesser tubulin staining than normal dendritic shafts. The domes of enlarged spines were moderately stained by tubulin antibody (Figure 3).
We speculate that in the vicinity of enlarged spines, packed normal shaft microtubules may be dispersed laterally as intraspinal microtubules, resulting in reduced staining of dendritic shaft microtubules. This lateral invasion of microtubules may cause spine enlargement. In negative controls using mouse serum as the primary antibody, there was almost no staining of microtubules. A staining defect seen in GFP-transfection or tubulin staining in an enlarged spine may be concentrated PSD-95, because in the enlarged spines delineated by GFP, staining defects and concentrated PSD-95 were sometimes colocalized (Figure 2).
This suggests that parts of the shaft microtubules participate in filling enlarged spines. The force of the lateral invasion of shaft microtubules may cause the structural changes leading to the formation of enlarged spines. This agrees with our previous electron microscopic data [8]. LTP-inducing stimulation introduced intraspinal microtubules emerged from the dendritic shaft to the postsynaptic membranes in acute hippocampal slices [8].
These results suggest that Map1b injection into the hippocampus may reverse spine shrinkage and have a positive effect on memory impairment in AD model mice.
Reduced postsynaptic density levels in the hippocampi of AD model mice were recovered by hippocampal MAP1B injection
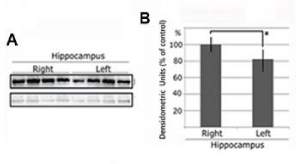
In both AD patients and AD model mice, including the model mice we used here [18], it has been reported that postsynaptic density is reduced [22]. As it has been reported that many proteins that localise to the postsynaptic site, that is, to dendritic spines, decrease in AD, the dendritic spine is a candidate for the initial location of AD pathology. We investigated whether administration of the Map1b-lentivirus chimera to the hemi-hippocampus of AD model mice causes recovery of the postsynaptic density. We tested several antibodies against PSD proteins, including PSD-95, and only an antibody against Homer Scaffolding Protein 1 (HOMER1) was successful, so we used this in subsequent investigations. HOMER1 localises to the postsynaptic site so we used and antibody against this to indicate postsynaptic densities. Seven days after injection of the Map1b-lentivirus chimera to the right hippocampus, during which time the MAP1B overexpression occurs, HOMER1 levels in the bilateral hippocampi were detected by western blotting. HOMER1 levels were recovered by hippocampal Map1b injection (Figure 4).
Discussion
The effectiveness of hippocampal MAP1B overexpression in rescuing memory impairment in AD model mice was transient. Large amounts of Amyloid (Aβ) may have been accumulating during this time in these AD model mice, as they have five mutations in APP and PS1. We speculate that the rescue effect of hippocampal MAP1B overexpression on memory impairment may be overcome by accumulation of Aβ. Taking into account the differences in the time frame and levels of amyloid beta production and the life spans of humans and the AD model mice used here, we speculate that this could be a new therapeutic strategy with considerable efficacy and duration for the treatment of AD.
To clarify the mechanisms of hippocampal MAP1B overexpression against memory impairment in normal neurons, we used primary cultures of wild type mice of the same strain as the transgenic AD model mice. We found that remarkable spine enlargement was induced by MAP1B overexpression. As recent reports strongly suggest the transportation of plasticity-promoting molecules to the stimulated postsynaptic membrane in normal memory formation [23, 24] and that spine loss or shrinkage is thought to be an important factor in AD pathology [3-5], this MAP1B-induced spine enlargement may be the key mechanism for rescuing memory impairment in AD.
To observe how spines are enlarged by MAP1B overexpression, enlarged spines and normal dendritic shafts were co-stained with tubulin antibodies (Figure 3). It was suggested that the MAP1B overexpression may worked for the spine enlargement via microtubules polymerization, because MAPs have the microtubules polymerizing effect. Our previous electron microscopy report [8], used acute hippocampal slices, LTP-producing stimulation, and microtubule-preserving fixation, demonstrated the emergence of stimulation-dependent intraspinal microtubules from dendritic shafts to postsynaptic membranes. This may help the interpretation of these findings, suggesting that dendritic shaft microtubules may participate in the formation of enlarged spines.
Here we have demonstrated that the reduction of the postsynaptic density protein HOMER1 in AD model mice is reversed by hippocampal MAP1B overexpression. This may support the finding that MAP1B overexpression enlarges spines in cultured neurons and that MAP1B overexpression improves memory impairment in AD model mice.
In the 1990s, two big discoveries in memory formation were made: One was that transportation of plasticity-promoting molecules to the postsynaptic membrane is essential for memory formation [23, 24]. However, this transportation must go only to the stimulated synapse [25]. The other is that memory formation is associated with transcriptional activation, indicating that signals need to be transported from the synapse to the nucleus [26].
The reports that Amyloid-β peptide binds to MAP1B [27], impaired hippocampal long-term potentiation in MAP1B-deficient mice [28] may support our findings.
Conclusion
The findings of this study suggest the possibility that a new therapy for AD, using hippocampal MAP1B overexpression, may not only restore shrunken spines but also restore the bidirectional transport between the stimulated postsynaptic membrane and the nucleus.
We thank Dr S. Okabe for kind gift of PSD-95-YFP, Hongo, Bunkyoku, 113-0033 Tokyo, Japan, Graduate School of Medicine and Faculty of Medicine, The University of Tokyo. We thank H. Shirasaki in the Division of Gene Expression Mechanism, Institute for Comprehensive Medical Science, Fujita Health University for technical support. We also thank Edanz Group (www.edanzediting.com/ac) provided English language editing services for a draft of this manuscript. We also thank Ms. H. Ohtsubo for illustration. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to declare.
- Riek R, Eisenberg DS (2016) The activities of amyloids from a structural perspective. Nature 539: 227-235. Link: https://goo.gl/pBQH2x
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, et al. (1989) Accumulation of abnormally phosphorylated x precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res 477: 90–99. Link: https://goo.gl/bdu2nW
- Scheff SW, Price DA (1993) Synapse loss in the temporal lobe in Alzheimer's disease. Ann Neurol 33: 190–199. Link: https://goo.gl/r5ha62
- Tsai J, Grutzendler J, Duff K, Gan WB (2004) Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nature Neurosci 7: 1181–1183. Link: https://goo.gl/UF44Rt
- Knobloch M, Mansuy IM (2008) Dendritic spine loss and synaptic alterations in Alzheimer's disease. Mol Neurobiol 37: 73–82. Link: https://goo.gl/W9Zj6f
- Dent EW (2017) Of microtubules and memory: implications for microtubule dynamics in dendrites and spines. Mol. Biol. Cell 28:1–8. Link: https://goo.gl/SrL9rN
- Sharma S, Ganesh T, David GI, Kingston DGI, Bane S (2007) Promotion of tubulin assembly by poorly soluble taxol analogs. An Biochem 360: 56–62. Link: https://goo.gl/cSxxhM
- Mitsuyama F, Niimi G, Kato K, Hirosawa K, Mikoshiba K, et al. (2008) Redistribution of microtubules in dendrites of hippocampal CA1 neurons after tetanic stimulation during long-term potentiation. Ital J Anat Embryol 113:17–27. Link: https://goo.gl/cVjZJb
- Mitsuyama F, Futatsugi Y, Okuya M, Kawase T, Karagiozov K, et al. (2012) Stimulation-dependent intraspinal microtubules and synaptic failure in Alzheimer's disease: a review. Int J Alzheimer’s Dis 519682. Link: https://goo.gl/6nmtyU
- Mitsuyama F, Futatsugi Y, Okuya M, Karagiozov K, Kato Y, et al. (2008) Microtubules to form memory. Ital J Anat Embryol 113:227–236. Link: https://goo.gl/va7SQL
- Mitsuyama F, Futatsugi Y, Okuya M, Karagiozov K, Peev N, et al. (2009) Amyloid beta: A putative intra-spinal microtubule-depolymerizer to induce synapse-loss or dentritic spine shortening in Alzheimer's disease. Ital J Anat Embryol 114:109–120. Link: https://goo.gl/ghRoit
- Gu J, Firestein BL, Zheng JQ. (2008) Microtubules in dendritic spine development. J Neurosci 28:12120–12124. Link: https://goo.gl/FEB45w
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. (2008) Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci 28: 13094–13105. Link: https://goo.gl/W688SX
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, et al. (2009) Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61: 85–100. Link: https://goo.gl/ah1KFm
- Tortosa E, Montenegro-Venegas C, Benoist M, Härtel S, González-Billault C, et al. (2011) Microtubule-associated protein 1B (MAP1B) is required for dendritic spine development and synaptic maturation. J Biol Chem 286: 40638-40648. Link: https://goo.gl/zMZftE
- Bassell GJ, Warren ST. (2008) Fragile X Syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60: 201–214. Link: https://goo.gl/BcZvvy
- Hirokawa N. (2006) mRNA transport in dendrites: RNA granules, motors, and tracks. J Neurosci 26: 7139–7142. Link: https://goo.gl/yYMtZk
- Oakley H, Cole SL, Logan S, Maus E, Shao P, et al. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci 40: 10129-10140. Link: https://goo.gl/1eCtFK
- Hayashi A, Asanuma D, Kamiya M, Urano Y, Okabe S. (2016) High affinity receptor labeling based on basic leucine zipper domain peptides conjugated with pH-sensitive fluorescent dye: Visualization of AMPA-type glutamate receptor endocytosis in living neurons. Neuropharmacol. 100: 66-75. Link: https://goo.gl/eguxFH
- Jiang M, Chen G. (2006) High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc 1: 695-700. Link: https://goo.gl/K13pLh
- Mitsuyama F, Kanno T (1993) Localization of Ca (2+)-calmodulin to the kinetochore of C6 glioma cells: an investigation of the anti-tumour effects of calmodulin antagonists in the treatment of brain tumours. Neurol Res 15: 131-135. Link: https://goo.gl/3iAphp
- Gong Y, Lippa CF. (2010) Review: disruption of the postsynaptic density in Alzheimer's disease and other neurodegenerative dementias. Am J Alzheimers Dis. 25: 547-555. Link: https://goo.gl/Sr9MaJ
- Liao D, Hessler NA, Malinow R. (1995) Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375: 400–404. Link: https://goo.gl/azKLuJ
- Strack S, Choi S, Lovinger DM, Colbran RJ. (1997) Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem 272: 13467–13470. Link: https://goo.gl/JqNaum
- Barrett AB, Billings GO, Morris RGM, Van Rossum MCW. (2009) State based model of long-term potentiation and synaptic tagging and capture. PLoS Comp Biol. 5: e1000259. Link: https://goo.gl/9BA7kN
- Alberini CM, Ghirardi M, Huang YY, Nguyen V, Kandel ER. (1995) A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann NY Acad Sci 758: 261–286. Link: https://goo.gl/iUYJsG
- Gevorkian G, Gonzalez-Noriega A, Acero G, Ordoñez J, Michalak C, et al. (2008) Amyloid-beta peptide binds to microtubule-associated protein 1B (MAP1B). Neurochem Int 52: 1030-1036. Link: https://goo.gl/nkJg3p
- Zervas M, Opitz T, Edelmann W, Wainer B, Kucherlapati R, et al. (2005) Impaired hippocampal long-term potentiation in microtubule-associated protein 1B-deficient mice. J Neurosci Res 82: 83-92. Link: https://goo.gl/39DKBk

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
