Annals of Alzheimer's and Dementia Care
Pilot experimental study; Effect of environmental stimulation consisting of sound with high-frequency components, aromas, and light exposure from organic light-emitting diodes (OLEDs) toward rest-activity rhythm in institutionalized patients with dementia
Yu Kume1*, Motoshi Tanaka2 and Katsutoshi Saito3
2Department of Mathematical Science and Electrical-Electronic-Computer Engineering, Graduate School of Engineering Science, Akita University, Japan
3Saikatu Inc., Akita, Japan
Cite this as
Kume Y, Tanaka M, Saito K (2022) Pilot experimental study; Effect of environmental stimulation consisting of sound with high-frequency components, aromas, and light exposure from organic light-emitting diodes (OLEDs) toward rest-activity rhythm in institutionalized patients with dementia. Ann Alzheimers Dement Care 6(1): 019-025. DOI: 10.17352/aadc.000024Copyright
© 2022 Kume Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Environmental stimulation is expected to have a positive impact on night sleep, psychological or functional states in dementia. The objective of this pilot study was to examine the effects of environmental stimulation consisting of sounds with high-frequency components, aromas, and light exposure from organic light-emitting diodes (OLEDs) to create comfortable living spaces for persons with dementia. Subjects (n =6) were recruited from elderly persons admitted to a single nursing home in Akita Prefecture, Japan, between August and September 2020. The night-time sleep state, the rest-activity rhythm, and the light exposure of the subjects living in environments with or without environmental stimulation consisting of sounds with high-frequency components, aromas, and light exposure from OLEDs were measured for 46.3 consecutive days using wrist activate devices under free-living conditions in a nursing home. In a period of environmental stimulation depending on the presence or absence of sounds with high-frequency components, reduction of the fragmented rest-activity rhythms was significantly observed in the subjects (p < 0.05). However, changes in the night-time sleep state had no significant difference during the study period. In conclusion, these preliminary results suggest that future examinations are warranted not only to inform effective or comfortable living conditions for elderly persons with dementia but also to improve the disruption of rest-activity rhythms in persons with dementia.
Introduction
Environmental stimulation, including light exposure, sounds, and aromas, is thought to have a positive influence on sleep-wake patterns [1,2], psychological behaviors [3,4] and daily activity functioning [5] in persons with dementia. Light therapy studies examining patients with dementia have reported that exposure to a 1,000-lx blue-enriched light every morning at 08:30 h for 30 min contributes to an improvement in daytime sleepiness, sleep disturbance, and depression after one week when evaluated using the Epworth Sleepiness Scale and Montgomery–Åsberg Depression Rating Scale [1]. Similar to the objective of the present study, a pilot study comparing regular ward lighting (5,500 K, 333 lx) and experimental lighting (14,000 K, 381 lx) in dayrooms showed that the experimental lighting was well received and that a 23% reduction in sleep latency (time in bed until the first 20 min of sleep) and a 6.6% increase in sleep efficiency were observed in persons with dementia [2]. Moreover, some environmental intervention studies have suggested that the utilization of sound or aroma stimulation in a nursing home can contribute to a reduction in agitation in residents with dementia [3] and significant improvements in Behavioral and Psychological Symptoms of Dementia (BPSD), as scored using the Neuropsychiatric Inventory (NPI). So far, the available information on daily environmental interventions does not suggest any serious adverse events and implies a mild sedative in persons with dementia. However, the effects of environmental stimulation on Rest-Activity Rhythm (RAR) parameters in persons with dementia have not been well documented.
Therefore, we aimed to clarify the effects of environmental stimulation combining sound with high-frequency components, aromas, and light exposure from organic light-emitting diodes (OLEDs) to create comfortable living spaces for persons with dementia. The hypothesis of our initial small, preliminary study was that environmental stimulation consisting of sounds with high-frequency components, aromas and light exposure from OLEDs might help to reduce fragmented RAR in residents with dementia. In addition, we examined the influence of the presence or absence of sounds with high-frequency components among the environmental stimuli during the study period.
Methods
Subjects
The study was conducted between August and September 2020. All institutionalized elderly persons with dementia (n = 8) living in a single nursing home (Community-Care Ouchi) in Akita Prefecture, Japan, were enrolled. The Clinical Dementia Rating (CDR) was applied to assess the severity of dementia in each participant and the results were classified as follows: CDR0, no dementia; CDR0.5, possible dementia; CDR1, mild dementia; CDR2, moderate dementia; and CDR3, severe dementia [6,7]. The inclusion criteria for the present study were as follows: (i) presence of dementia as diagnosed by a medical doctor specializing in geriatrics based on the resident’s medical records and the results of a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [8], (ii) no use of a benzodiazepine for sleep disturbances within the study period, and (iii) absence of substance-related disorders and mental retardation. The exclusion criteria were as follows: (i) difficulty wearing the wrist device continuously because of skin irritation, (ii) absence of complete data collection throughout the study period, or (iii) study dropout because of a worsening of the subject’s physical condition.
This study was approved by the ethics committee of Akita University and was performed by the Declaration of Helsinki II. To ensure that the experiment’s protocol conformed with international ethical standards, the ethics and methods of previously reported biological rhythm research studies were adopted [9]. After the agreement was obtained from all the subjects, demographic data including age, sex, and independence of daily activities was collected from the facility’s records.
Intervention
Light stimulation was performed using OLEDs. We fixed a board of 64 OLEDs’ panels (single panel size [W×H×D], 92.4×92.4×4.3 mm; weight, 42 g; OLE-P0909-G2B, Pioneer) to the wall of the facility’s dayroom (the space where all the residents gathered during the daytime; 63.07 m2 of floor space), as shown in Figure 1. The maximum luminosity (cd/m2) of a single panel was 2,000 cd/m2. The color temperature (K) ranged from 2,000 K to 6,000 K between 06:00h and 12:00h and from 6,000 K to 2,000 K between 12:00h and 22:00h and was altered using a programmable system. Additionally, a single bedside lighting device was installed in each participant’s living space.
For aroma stimulation, two aromas were automatically diffused in the dayroom using two diffusers (Air FLOW, COMMONS Co., Ltd.). Essential oil fragrances consisting of 100% rosemary camphor oil and 100% lemon oil were emitted in the morning (05:00-08:00 h), while 100% lavender oil and 100% sweet orange oil were emitted in the evening (18:00-21:00h). High-resolution sound stimulation with 96 kHz sampling frequency was performed during the day using a sound system (KooNe, Victor Entertainment Corp.) comprised of a set-top box (STB), a four-channel amplifier (size [W×H×D], 255×96×289 mm), and four ceiling-mounted speakers (size [W×H×D], 241.2×37×55.2 mm). Natural environmental sounds, including the sounds of birds, natural streams, and the wind in trees, were used for sound stimulation. The sound stimulation was broadcasted 24-h daily.
Experimental procedure
The subjects took part in a total of 5 study phases (1,112 h or 46.3 d in total) consisting of a baseline period without stimulation (Baseline), intervention phase I consisting of a combination of light, sound, and aroma stimulation (Phase I), intervention phase II consisting of a combination of light and aroma stimulation (Phase II), intervention phase III consisting of a combination of light, sound, and aroma stimulation (Phase III), and finally a follow-up period without stimulation (Follow-up). The Baseline period lasted 167 h (approximately 6.9 d), Phase I lasted 176h (approximately 7.3 d), Phase II lasted 153 h (approximately 6.4 d), Phase III lasted 448 h (approximately 18.7 d), and the Follow-up period lasted 168 h (7 d). The subjects followed their ordinary daily life activities while living in these environmental settings.
Outcome measurements
The Actiwatch Spectrum Plus (AW-SP; Philips Respironics, Inc.) was used to assess changes in sleep parameters during the night, RAR variables, and the illuminance (lx) of the white or blue light to which each participant was exposed and for how long. The subjects were instructed to wear an AW-SP device on the non-dominant wrist throughout the study period without removal. The collected AW-SP data, including the activity counts and night-time sleep parameters, were estimated using Actiware version 6.09 (Philips Respironics, Inc.). The collected sleep parameters consisted of the average Sleep Efficiency (SE) (%), the average walking time (AT) (min), and the average total sleep time (TST) (min) during each phase. Nonparametric RAR variables were also calculated using the AW-SP data. The RAR variables consisted of Internal Stability (IS), intra-daily variability (IV), and Relative Amplitude (RA) [10,11]. The IS variable reflects RAR synchronization in response to stable environmental stimuli and ranges in value from zero for normal distribution noise to one for complete synchronization. The IV variable reflects the fragmented state of the RAR and ranges in value from zero for non-fragmented RAR to two for fragmented RAR. Lastly, the RA variable reflects the relative proportion between the most active 10-h span (M10) and the least active 5-h span (L5) over the average 24-h profile and ranges in value from zero for a low amplitude to one for a high amplitude. Furthermore, the light exposure during the study period was recorded for each participant as the average white-light exposure (in lx) from OLEDs between 06:00h and 22:00h over the average 24-h profile and the average blue-light exposure (in lx) between 06:00h and 22:00h over the average 24-h profile. We also computed additional light variables including the mean white-light levels per one-minute interval (in lx), the minutes spent over 1000 lx (in min) and the minutes spent over 2000 lx (in min) between 06:00h and 22:00h during each phase. The values reported in Shochat Martin, et al.’s study [12] were used as references.
Statistical analysis
The normal distributions of all the variables including the sleep parameters (SE, AT, and TST), the RAR variables (IS, IV, RA, L5, and M10), and the light variables (average white- or blue-light exposure, mean white-light levels per one-minute interval, and minutes spent over 1000 lx or over 2000 lx) were first confirmed using the Shapiro-Wilk test. The sleep parameters, RAR variables, and light variables were compared using repeated analysis of variance (rep. ANOVA) with the Tukey honestly significant difference test used as a post hoc test. Finally, activity plots and light plots were created for each study phase to obtain an overall view of the average 24-h profile, thereby enabling objective comparisons of RAR and light exposure and the discernment of any changes. SPSS Version 24.0 for Windows (SPSS Inc., Chicago. IL, USA) was used for the analysis, and the level of significance was set at p = 0.05.
Results
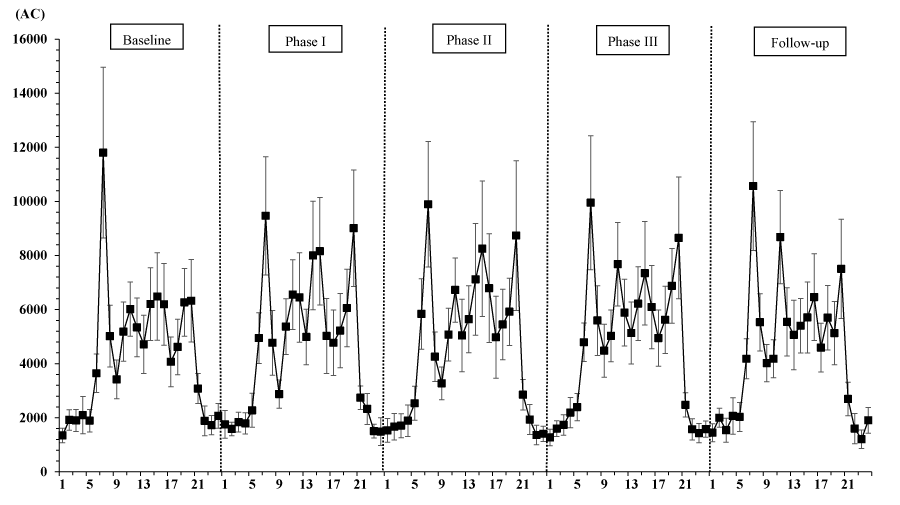
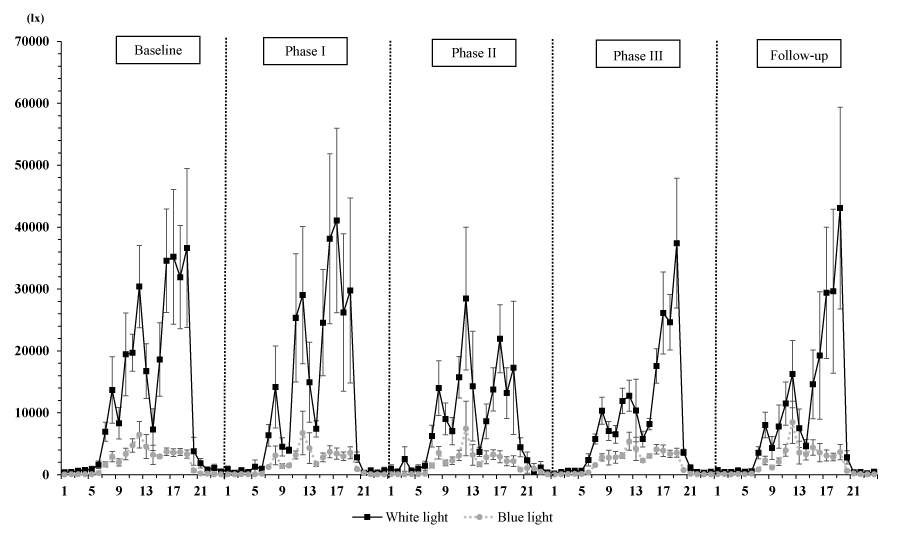
The final sample included 6 residents with dementia. The average (standard deviation [SD]) age, sex ratio (female/male) and average (SD) CDR score were 83.7 (4.5) years, 4/2, and 1.3 (0.9), respectively (CDR0.5, n = 1; CDR1, n = 4; CDR3, n = 1). Table 1 shows the sleep measurements, RAR variables, and light variables. A rep. ANOVA showed that IS and IV, among the RAR variables, and the minutes spent over 1000 lx and 2000 lx differed significantly among the study phases (IS: F [4, 20] = 4.15, p = 0.013; IV: F [4, 20] = 5.32, p = 0.004; min > 1000 lx: F [4, 20] = 14.2, p = 0.00001; min > 2000 lx: F [4, 20] = 6.0, p = 0.002). According to multiple comparisons using the Tukey honestly significant difference test, the IS during Phase III was significantly lower than that during Phase II (p = 0.012) or the Follow-up period (p = 0.033), while the IVs during Phase I (p = 0.002) and during Phase III (p = 0.043) were significantly lower than that during the Baseline period. Regarding the light variables, the amount of time spent over 1000 lx during Phase III was significantly longer than that spent during the other phases (p < 0.05), and the amount of time spent over 2000 lx was significantly longer during Phase III than during the Baseline, Phase II, and Follow-up periods (p < 0.05). However, all the sleep parameters and the RAR variables, with the exception of IS and IV, as well as the other light variables (white-light exposure and mean light exposure per one-minute interval) did not differ significantly according to the study phase (p > 0.05). In addition, Figure 2 shows the 24-h activity plots for each phase, and Figure 3 shows the light-exposure plots divided into white- or blue-light over the 24-h profile for each phase.
Discussion
The result demonstrated that in a period intervened by environmental stimulation of sounds with high-frequency components, aromas and light exposure from OLEDs, a significant reduction in fragmented RAR can be observed depending on the presence or absence of sounds with high-frequency components. Additionally, RAR synchronization in response to stable environmental stimuli differed significantly from the type of environmental intervention. However, improvements in sleep parameters were not observed during the study period.
Of the three environmental stimuli examined in the present study, the 24-h white or blue light exposure (in lx) did not differ significantly among the study phases (Table 1 and Figure 3), and the results for RAR and light exposure suggest that light exposure from OLEDs did not influence the RAR in the subjects in response to a lack of light-exposure in the present experimental setting. Similar to our study’s methodology, Shochat, et al.’s study (2000) examined 77 institutionalized elderly individuals (mean±SD age, 85.8±7.3 years; 58 women and 19 men; no dementia, n = 3; mild dementia, n = 9; moderate dementia, n = 10; severe dementia, n = 44) and reported that light levels with more minutes spent over 1,000 lx or over 2,000 lx (mean±SD light exposure during the daytime, 485±761 lx; range, 43 to 3,565 lx) were significantly associated with the number of night-time awakenings (coefficient = -0.006, p = 0.015) when their data was examined using a multiple regression model (i.e., higher light levels predicted fewer awakenings during the night regardless of dementia severity). Moreover, a multiple regression model comparing light levels and circadian rhythms of activity suggested that the times (in min) spent exposed to light stimuli over 1000 lx and over 2000 lx were significant predictors (>1000 lx: coefficient = 0.016, p = 0.007; >2000 lx: coefficient = 0.016, p = 0.007) of the acrophase of activity, as reflected by the timing of the peak activity during the day. Although these findings cannot be directly compared with the results for light variables in the present study because of differences in the experimental settings (i.e., differences in environmental stimuli or experimental durations) and the use of different circadian variables (e.g., RAR variables), our mean light level per one-minute interval (mean±SD light exposure per one-minute interval: 295.4±131.0 lx during the Baseline period, 275.5±184.6 lx during Phase I, 191.8±99.9 lx during Phase II, 197.6±70.6 lx during Phase III, and 211.9±193.8 lx during Follow-up period) was substantially lower throughout all the phases (Table 1). The minutes of light exposure over 1000 lx and over 2000 lx also differed significantly among the study phases, although these values were dependent on the duration of each phase (Baseline period, 6.9 d; Phase I, 7.3 d; Phase II, 6.4 d; Phase III, 18.7 d; Follow-up period, 7 d) (Table 1). Furthermore, some studies examining illumination levels have reported that even a relatively low light intensity of 180 lx can influence the circadian pacemaker in humans [13], and institutionalized elderly persons with mild or moderate dementia spent a median of 9 min exposed to light over 1000 lx, while those with severe dementia spent only 1 min exposed to light over 1000 lx [14]. Thus, these findings warrant further investigation of illumination levels in care facilities.
Based on the above findings regarding light exposure and persons with dementia, a comparison of the phases in the present study supports the hypothesis that fragmented RAR can be reduced by environmental stimulation with sounds with high-frequency components and aromas. Although the presently available information is insufficient to support a causal relationship between circadian variables and general effects of sounds with high-frequency sounds and aromas, some studies of elderly persons with dementia have reported the following effects: in a review of randomized controlled trials (RCTs) examining aromatherapy for persons with dementia [15], the results from one RCT study examining 72 patients with dementia revealed a significant effect of 4 weeks of aromatherapy on neuropsychiatric symptoms as indexed in The NPI total score (effect, -15.8; 95% confidence interval, -24.37 to -7.22, p = 0.004) [16]. Concerning the relationship between neuropsychiatric symptoms in persons with dementia and RAR variables, our previous study with 77 subjects (49 residents with dementia, 28 residents without dementia) recruited from five facilities reported that an increase in fragmented RAR was significantly associated with behavioral and psychological symptoms (as evaluated using the dementia behavior disturbance [DBD] scale) among nursing home residents (fixed effect of multi-level models, coefficient [standard error, SE] = 39.73 [16.28], p = 0.02) [17]. In the present study, a significant decrease in fragmented RAR was found between The baseline period and Phase I (and between the Baseline period and Phase III), whereas a tendency for subjects to experience an increase in IV when shifting from Phase I (exposure to light, aroma, and sound stimuli) to Phase II (exposure to light and aroma stimuli only) was also observed (Table 1, Figure 2).
Interestingly, these results suggest a new perspective on the associations among environmental stimuli, RAR variables, and neuropsychiatric symptoms in persons with dementia and raise the possibility that sounds with high-frequency components might contribute to a reduction in fragmented RAR among institutionalized elderly persons with mild or moderate dementia. Limited information is available regarding the mechanism or the effects of sounds with high-frequency components in humans; a basic study reported that changes during sound stress of the circadian rhythm, which is its basic mechanism to the RAR patterns, were significantly observed in the heart rate variability (HRV) changes [18], but a paucity of material is available on a correlation between the circadian rhythm or the RAR mechanism and sound stimulation. According to literature related to the effect of sound stimulation, sound levels (auditory threshold 8,000Hz) were associated with the number of apneas and hypopneas per hour of sleep, the oxygen desaturation index, and decreased oxygen saturation in 43 patients aged 34 to 74 years with Obstructive Sleep Apnea (OSA) [19], as well as a decrease in agitation in institutionalized elderly persons with dementia [20]. However, the effect of sounds with high-frequency components on RAR patterns and sleep states in persons with dementia remains unclear, although studies are in progress to characterize these concerns. In relation to changes in RAR synchronization (e.g., IS value in Table 1) in persons with dementia, Smagula, et al. (2019) conducted a systematic review and reported that nursing-home residents with dementia have lower IS values (reflecting less stability across days) and that the stability of RAR in persons with dementia can be characterized as a state of flux, depending on environmental changes [21].
The limitations of our pilot study were the small number of persons with dementia, the absence of a control cohort, and the inclusion of subjects from a single facility only. Sufficient information for psychological or behavioral symptoms (such as hallucination, depression, agitation, et al.) in dementia cannot be also obtained from the pilot study, but further examinations regarding the BPSD as associated with sleep-wake patterns in dementia [22] need to be carried out. As well, future research should examine patients with or without dementia from multiple facilities to confirm if environmental stimulation (especially, the presence or absence of sounds with high-frequency components sound) alters rest-activity rhythms in dementia.
Conclusion
The results of our preliminary study suggest that an environmental approach utilizing sounds with high-frequency components at the basis of exposure to light, aroma, and sound stimuli could enable a decrease in fragmented RAR among institutionalized elderly persons with mild to moderate dementia. In addition, unstable RAR in institutionalized elderly persons with mild to moderate dementia might be associated with variations in environmental conditions, including light, aroma, and sound stimuli.
Author contributions
M.T. and K.S. designed this study and worked to set up the apparatus related to stimulation in living environmental situations. Y.K. analyzed the collected data for the study and wrote the article.
Institutional review board statement
This study was approved by the ethics committee of Akita University and was performed in accordance with the Declaration of Helsinki II. To ensure that the experiment’s protocol conformed with international ethical standards, the ethics and methods of previously reported biological rhythm research studies were adopted [9].
Informed consent statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data availability statement
We cannot publicly provide individual data due to subjects’ privacy, as specified by the ethics committee.
We wish to thank all the subjects and Mr. Takashi Ogasawara, chief-director and staff for Nonprofit Organization (NPO) Community-Care Ouchi in Yurihonjo City.
- Ambar Akkaoui M, Paquet C, Geoffro PA (2020) Bright light therapy improved sleep disturbances in a patient with dementia with Lewy bodies. Psychogeriatrics 20: 124-125. Link: https://bit.ly/3Cz7uBZ
- Hornick TR, Higgins PA, Duffy ME, Beers WW, Figueiro MG (2015) Using Light to Manage Sleep-Wake Issues in Patients With Dementia. Fed Pract 32: 42-45. Link: https://bit.ly/3hUZzpb
- Bulsara C, Seaman K, Steuxner S (2016) Using sound therapy to ease agitation amongst persons with dementia: a pilot study. Aust Nurs Midwifery J 23: 38-39. Link: https://bit.ly/3IV9U0b
- Fujii M, Hatakeyama R, Fukuoka Y, Yamamoto T, Sasaki R, et al. (200) Lavender aroma therapy for behavioral and psychological symptoms in dementia patients. Geriatr Gerontol Int 8: 136-138. Link: https://bit.ly/34s4tGZ
- Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P (2014) Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev CD003946. Link: https://bit.ly/3tKoLEe
- Hughes CP, Berg L, Danziger WL, Coben LA, et al. (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140: 566-572. Link: https://bit.ly/3hPogTJ
- Paavilainen P, Korhonen I, Lotjonen J, Cluitmans L, Jylha M, et al. (2005) circadian activity rhythm in demented and non-demented nursing-home residents measured by telemetric actigraphy. J Sleep Res 14: 61-68. Link: https://bit.ly/3pRBO5D
- Dilip VJ, Jeffrey AL, David F, Roger P (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC, London, England, 2013 American Psychiatric Association 591-643. Link: https://bit.ly/379mMSd
- Portaluppi F, Smolensky MH, Touitou Y (2010) Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int 27: 1911-1929. Link: https://bit.ly/3vQaU1S
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, et al. (1999) Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int 16: 505-518. Link: https://bit.ly/3I2uGda
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF (1990) Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry 27: 563-572. Link: https://bit.ly/35ZDiUn
- Shochat T, Martin J, Marler M, Ancoli-Israel S (2000) Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res 9: 373-379. Link: https://bit.ly/3CvzQNE
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA (1996) Dose-response relationships for resetting of human circadian clock by light. Nature 379: 540-542. Link: https://bit.ly/3tOzjT4
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, et al. (1997) Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep 20: 18-23. Link: https://bit.ly/37fWG06
- Thorgrimsen L, Spector A, Wiles A, Orrell M (2003) Aroma therapy for dementia. Cochrane Database Syst Rev CD003150. Link: https://bit.ly/3vNxuZ7
- Ballard CG, O'Brien JT, Reichelt K, Perry EK (2002) Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry 63: 553-558. Link: https://bit.ly/3J3Bf0o
- Saito Y, Kume Y, Kodama A, Sato K, Yasuba M (2018) The association between circadian rest-activity patterns and the behavioral and psychological symptoms depending on the cognitive status in Japanese nursing-home residents. Chronobiol Int 35: 1670-1679. Link: https://bit.ly/37kxllP
- Takeuchi H, Enzo A, Minamitani H (2001) Circadian rhythm changes in heart rate variability during chronic sound stress. Med Biol Eng Comput 39: 113-117. Link: https://bit.ly/3tHEOmf
- Vorlova T, Dlouha O, Kemlink D, Sonka K (2016) Decreased perception of high frequency sound in severe obstructive sleep apnea. Physiol Res 65: 959-967. Link: https://bit.ly/35B7OEs
- Joosse LL (2012) Do sound levels and space contribute to agitation in nursing home residents with dementia? Res Gerontol Nurs 5: 174-184. Link: https://bit.ly/3KorXvY
- Smagula SF, Gujral S, Capps CS, Krafty RT (2019) A Systematic Review of Evidence for a Role of Rest-Activity Rhythms in Dementia. Front Psychiatry 10: 778. Link: https://bit.ly/3hTTZDk
- Cho E, Kim S, Hwang S, Kwon E, Heo SJ, et al. (2021) Factors Associated With Behavioral and Psychological Symptoms of Dementia: Prospective Observational Study Using Actigraphy. J Med Internet Res 23: e29001. Link: https://bit.ly/3Cpf2qS

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
