Annals of Alzheimer's and Dementia Care
The cost and benefit of targeting amyloid plaques to treat alzheimer’s disease
Marisa E Franklin1 and Glen A Franklin2*
2Hiram C Polk Jr. MD Department of Surgery, University of Louisville School of Medicine, Louisville, Kentucky, USA
Cite this as
Franklin ME, Franklin GA (2023) The cost and benefit of targeting amyloid plaques to treat alzheimer’s disease. Ann Alzheimers Dement Care 7(1): 008-013. DOI: 10.17352/aadc.000027Copyright
© 2023 Franklin ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Alzheimer’s Ddisease (AD) is now the seventh leading cause of death in the United States. This incurable and fatal disease is becoming an increasingly concerning medical diagnosis. One feature of Alzheimer’s disease is a build-up of the peptide amyloid-beta in the brain that progressively interferes with the patient’s ability to retain memory and perform mental tasks. One of the newest strategies to combat AD is to target the excess amyloid-beta. Several new drugs have been in stages of clinical trials, with both Lecanemab and Aducanumab recently being deemed a scientific breakthrough by gaining Food and Drug Administration (FDA) approval. This review will address the various strategies scientists have taken to target amyloid-beta, discuss the cost versus benefit of the drugs, and attempt to place this information in context with this significant public health crisis.
Introduction
Alzheimer’s disease is an incurable neurological disease that largely affects the older generations (ages 65 and up). One of AD’s hallmark symptoms is dementia, which widely affects the patient’s cognitive ability, such as memory loss, inability to problem solve, and decreased function to communicate with others. Because Alzheimer’s disease is dependent on age, it is becoming vastly more prevalent as humanity’s lifespan continues to rise. Roughly 11% of America’s older population experiences some degree of cognitive decline due to Alzheimer’s disease [1]. This is becoming a national health crisis amongst humanity’s older generation.
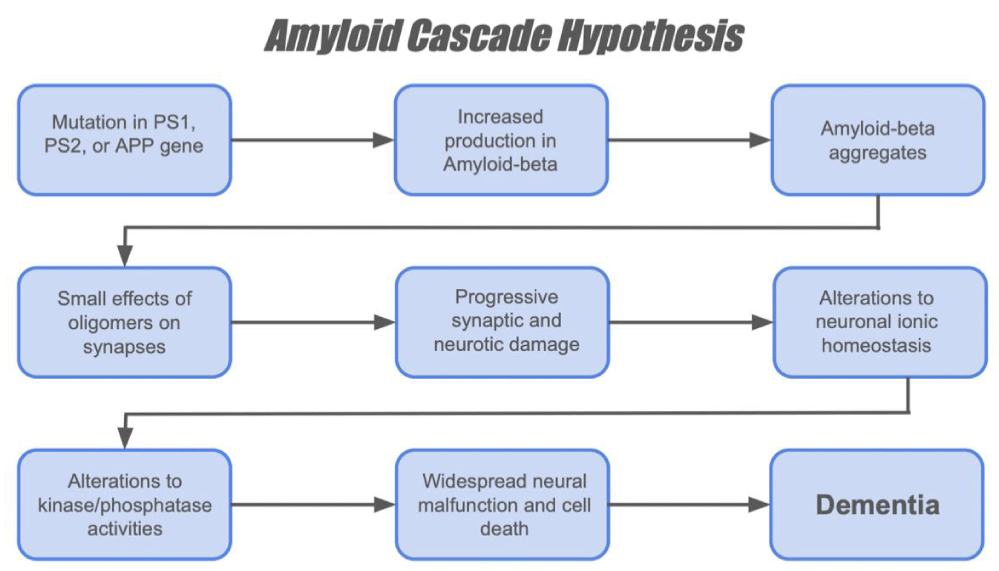
While there is no definitive answer for the cause of Alzheimer’s disease, many researchers look to its cardinal feature, the amyloid plaques, for solutions. The Amyloid Cascading Hypothesis is a popular theory behind the cause of AD. This hypothesis reveals how the small effects of a genetic mutation in the genes can snowball into Alzheimer’s disease. In short, when the APP, PS1, or PS2 gene mutates, amyloid-beta 42 is overproduced, which leads to a build-up and plaque formation. From there, the plaques start interfering with neurological activities, blocking neurons and intercepting messages. This is the commonly presumed pathway to the development of Alzheimer’s dementia (Figure 1) [2-5]. Alternate theories have suggested a much more complex etiology of disease development and progression [6] redefining a movement away from the amyloid cascade. As most cases of AD (> 95%) occur as sporadic AD, there are many triggers associated with genetic risk, environmental pressure, and multifactorial pathways to cognitive impairment. Amyloid-beta clearly represents a diagnostic feature of AD if not a role in etiology. The success with targeted antibodies has renewed enthusiasm for the amyloid cascade hypothesis.
Targeting amyloid-beta
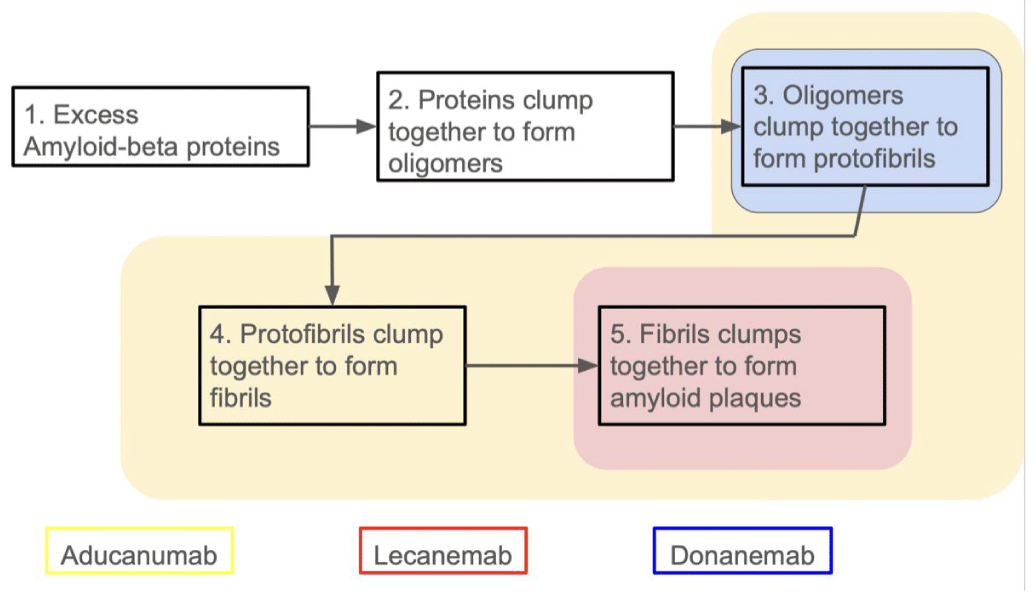
As mentioned before, Amyloid-beta is widely accepted as one of the main features, if not a main cause, of AD. This led it to become a main focus within the scientific community on the mad hunt for a cure. Because of the surge in interest targeting amyloid-beta, there has been a plethora of strategies that have resulted. Various conformations of amyloid-beta eventually lead to plaques being formed, and each one of the strategies that have been trialed implements a different method to attack the amyloid-beta, targeting different conformations.
Vaccines
One of the more obscure strategies was an emphasis on developing a vaccine. One of the first trials took place in 1999. The transgenic PDAPP mouse develops many of the same traits of Alzheimer’s disease, including the building up of amyloid plaque. Clinical trials showed that when younger mice were immunized with 42-amino-acid, which is the amino acid they identified as a main contributor to the build of amyloid plaque, there was a significant reduction in amyloid plaque later on in life. They also observed a smaller, but still significant, decrease in the plaque in mice they had immunized later in life [7]. This huge success launched the vaccine, now called AN-1792, into human trials; however, the results from the trials were less impressive. The clinical trial was abruptly halted when they noticed a rise in aseptic meningoencephalitis [8]. Despite the huge success found in trial mice, AN-1792 only produced a rate of 20% in the immune response to amyloid-beta. Follow-up trials on patients who received the vaccine were noted to see a decrease in amyloid plaque in patients who had an immune response; however, it is important that not all of the patients who received the vaccine had a substantial immune response. It’s also noted that the limited number of patients (n = 22) involved in the trial and limited knowledge at the time of the trial might have led to patients receiving the vaccine who initially were not predisposed to Alzheimer’s disease, therefore skewing the results [8,9].
Due to the perceived failure of the AN-1792 vaccine, minimal trials were conducted until last year when a Japanese study worked to immunize mice against senescent cells expressing Senescence-Associated Glycoprotein (SAGP) [10]. SAGP is largely affiliated with old-age diseases, one of which the study was targeting was Type II diabetes. An unexpected outcome of this trial was the immune response from the vaccine, which also targeted overexpressed glial cells in the brains of patients with Alzheimer’s disease. Mice who received this vaccine showed a reduction in amyloid plaques compared to those who received the placebo. The immunized mice also showed a decrease in abnormal behaviors related to Alzheimer’s disease, which is something that the AN-1792 vaccine was unable to accomplish [11]. This vaccine is still in the works, and major studies involving human trials have not been released. The limited success in amyloid removal has stimulated new interest in vaccine technology [12]. There are currently seven U.S. clinical trials underway utilizing vaccines to target amyloid-beta (ClinicalTrials.gov). Unfortunately, there are no comparison studies to review vaccine-related therapies versus antibody therapy for the reduction of amyloid plaques or the progression of disease.
Passive immunotherapies
Other more passive immunotherapies have put a massive spotlight on Alzheimer’s disease. Two new medications, Lecanemab and Aducanumab, target amyloid-beta and made headlines when they were both recently approved by the Food and Drug Administration (FDA). Despite there being numerous drugs in the process of clinical trials, the three forerunners of this race, Lecanemab, Donanemab, and Aducanumab, all utilize different mechanisms that attack the different stages of plaque buildup (Figure 2) [13-15].
Lecanemab was the most recent Alzheimer’s disease drug to be approved by the FDA. So far, it has proven to be more effective than Aducanumab, as well as having decreased side effects [16-18]. Lecanemab uniquely targets the formation of amyloid plaques rather than removing them. This monoclonal antibody binds to amyloid-beta soluble protofibrils. This bonding blocks plaques from forming. Clinical trials reveal that Lecanemab showed a promising 72% reduction in Alzheimer’s disease progression compared to the placebo [17].
Donanemab is the only leading Alzheimer’s disease drug in this class still in clinical trials that does not have FDA approval. Unlike Lecanemab, Donanemab targets the plaques themselves, binding to the N-terminal of stable amyloid plaques. This is significant because it tackles the problem that the immune system doesn’t recognize amyloid plaques as a threat. However, once Donanemab binds to the terminal, it signals the immune system to eliminate the plaques [19]. Donanemab’s biggest problem is how quickly the body forms anti-donanemab antibodies and its high development rate of Amyloid-Related Imaging Abnormalities (ARIA). Still, despite all of this, Donanemab still performed a 35% decrease in the progression of Alzheimer’s disease and 68% of patients participating in a 76-week trial saw a decrease in amyloid plague build-up [19-21].
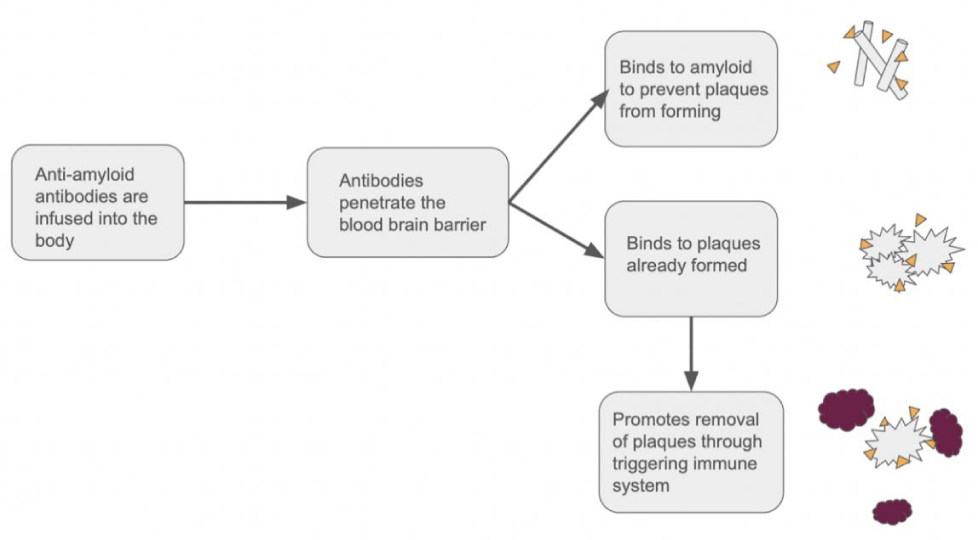
While both Lecanemab and Donanemab target specific conformations of amyloid plaque, Aducanumab targets multiple stages, binding to the residues 3-7, which is an epitope exposed in all conformations of amyloid-beta. It shows a high affinity to bind to oligomers, insoluble fibrillar, and amyloid plaques. This allows Aducanumab to not only attack plaques that have already formed but also prevent them from further forming. Through binding to this epitope, Aducanumab significantly increases the phagocytosis of the amyloid-beta complexes [22]. In Aducanumab, the final trial showed a promising 71% reduction in brain amyloid compared to the 4% shown in the placebo (Figure 3) [13].
Multiple trials are underway for each of these products. Cummings, et al. [23] provided an excellent review of the most recent drugs and trials for the anti-amyloid monoclonal antibodies. Table 1 (adapted from Cummings ref. 23) summarizes the FDA Phase II//III trials that have led to the approval process for Aducanumab [16,24-27] and Lecanemab [17,28] as well as the application process for Donanemab [21,29-33]. Aducanumab has completed a Phase I trial utilizing a subcutaneous dosing regimen which would illuminate the need for IV infusion. This was a small safety trial (n = 28), but the beginning of a process to look for easier forms of drug delivery which offers significant advantages to the patient and caregiver.
Photo-oxygenation
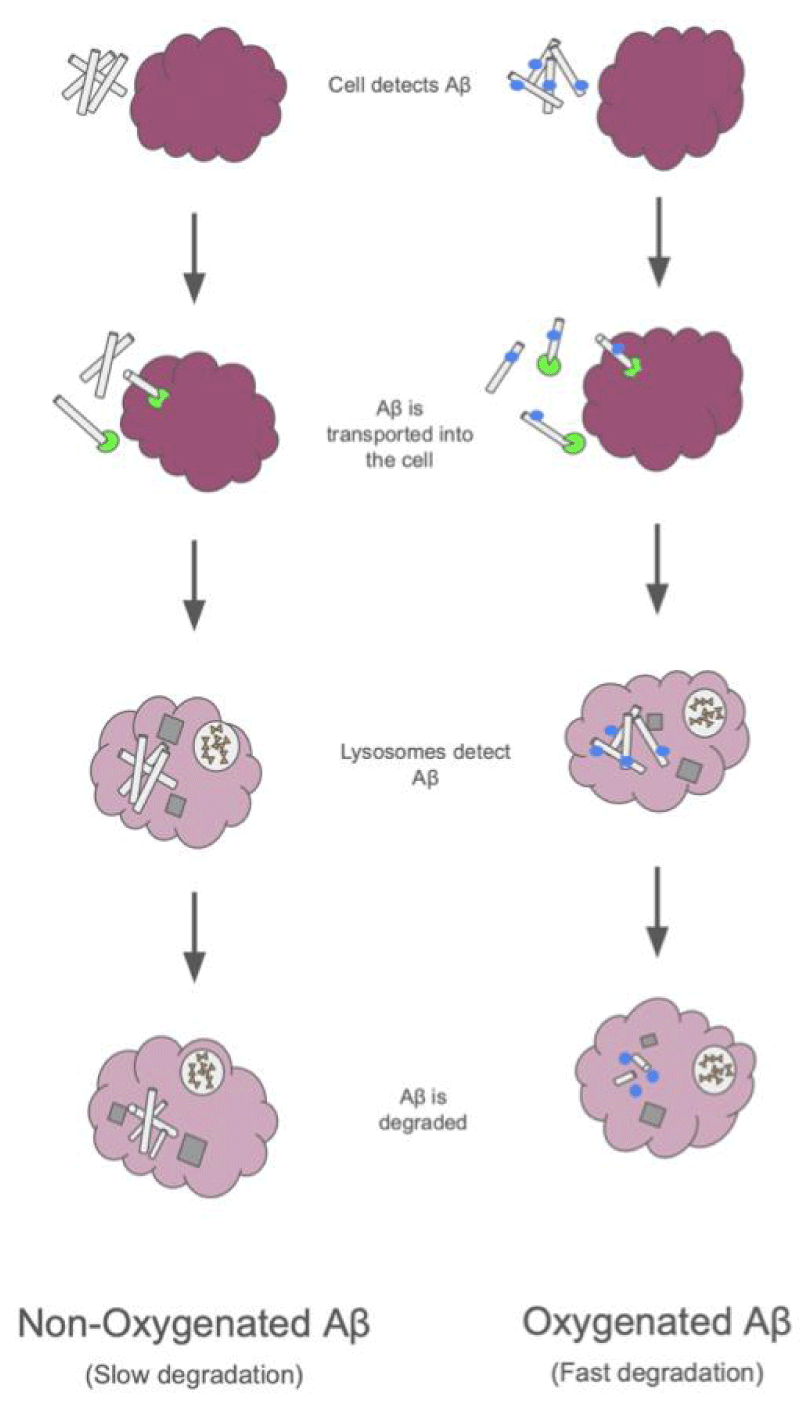
An additional strategy that specifically targets amyloid plaques is photo-oxygenation. Many of the immunotherapies listed above are larger molecules that have difficulty penetrating the semi-permeable barrier to the brain. Because of this, none of the aforementioned strategies have a very efficient delivery. This is why a small photocatalytic molecule called a BAP-1-based catalyst was developed. This catalyst only reacts when exposed to light and then attaches oxygen to the amyloid-beta, which reduces the neurotoxicity of the plaques [34]. What makes the photocatalyst unique is its high permeability across the blood-brain barrier, which increases the delivery efficiency when compared to other immunotherapies. This is a novel technique that is still in the process of development; however, it has been shown to be a faster process than other immunotherapies. This is still a strategy in the very early stages of development, and while the decrease in amyloid plaques in mice has been observed, it still has not moved on in human clinical trials [35] (Figure 4).
Cost VS. benefit analysis
There has been a lot of controversy surrounding passive immunotherapies and their rushed FDA approval. The main issue surrounding them is the huge price tag associated with them, especially Aducanumab. Aducanumab’s estimated price is as high as $84,000 (USD) annually, not including the extraneous costs such as travel to the hospital every four weeks to be administered and the follow-up MRIs that patients must undertake. However, the most likely projected cost will be an additional $56,000 annually. This number can be considered compared to other AD medications, such as Donepezil ($80 annually) and Memantine ($250 annually). While both of these medications only work to treat symptoms of AD rather than to stop the progression or even reverse the progression as Aducanumab promises, the price differences are striking [36,37].
A Markov state-transition model was conducted on Aducanumab to address the cost-efficiency of the drug. They estimated that the willingness to pay value was 100,000/QALY. Basically, this means that for every quality-adjusted life year (QALY) gained through treatment, patients would be willing to pay $100,000. Compared to the standard at-home care received by most mild cases of AD, treating mild AD for five years with Aducanumab would cost $383,080/QALY, significantly over the $100,000/QALY threshold estimated that most people would pay. Through calculations, it was estimated that the cost of moderate to severe AD would have to be more than $209,720 annually for the $56,000 price tag to be worth it. In addition, to meet the $100,000 threshold, Aducanumab would have to be priced at $22,800 annually [36].
Lecanemab is not much better, estimated to cost $26,500 annually. While conducting a similar study on comparing Lecanemab to normal at-home care, the Institute for Clinical and Economic Review (ICER) concluded that Lecanemab would have to be roughly between $8,900 – $21,500 per year in order to achieve the threshold that most consumers would hold the benefits gained from receiving Lecanemab as treatment [38]. ICER also compared the effects Lecanemab pricing would have not only through a healthcare sector perspective but also through a modified societal perspective. This perspective lowers the quality-adjusted years gained through the normal at-home care most AD patients receive because it also considers the quality of life that is reduced for the caretaker. When taking the burden of care on families into account, the difference between the cost-effectiveness of Lecanemab and normal supportive care does lower; however, it doesn’t lower it enough to make Lecanemab’s current listing price worth the benefits gained.
However, when assessing cost from an overall standpoint, these medications might save the healthcare system billions of dollars. With the rising rates of AD appearing in the older population, the US healthcare system is being strained to take in the influx of patients. The cost of AD on the healthcare system is nearing $1 trillion, putting a heavy economic burden on society [1]. While these anti-amyloid-beta drugs haven’t been on the market long enough to see the effects on the economy, studies previously done of other AD drugs, such as memantine, show that the total cost to society actually decreased [39]. This decrease occurs for various reasons, including fewer hospitalizations due to AD and an increase in caretakers’ ability to take on jobs. A true cost/benefit analysis cannot be performed since a reduction in the progression of disease is difficult to adjust on a cost accounting model as it only presumes that there is a decreased burden on the healthcare system. Moving towards a subcutaneous formulation of these drugs may further reduce costs theoretically by avoiding the need for scheduled infusions, provided the cost of the drug remains the same. Unfortunately, it is common for different dosing formulations to be provided at different costs which may negate the positive financial impact of a home subcutaneous dosing strategy. However, from a practical standpoint, the economic impact of AD on our society is real, its negative psychological effect on family/caregivers and the loss of function/joy from the patients certainly warrants a continued clinical investigation and development of novel treatments for this disease, albeit currently at a fairly high drug cost.
Conclusion
Alzheimer’s disease is a complex disease with a very poorly understood etiology and many failed treatments [40,41]. The past few decades have seen major leaps in treatment development, especially in disease modification, such as targeting amyloid plaques; however, there is plenty of room to grow. Many of these strategies can only be fully effective if they are administered in the early stages of Alzheimer’s disease. This challenges scientists to discover more effective measures to identify early signs of AD or the possible use of genetic testing for those predisposed to AD. Looking towards the future, scientists will look for more ways to combat this horrible disease and identify the etiology of its development. The true impact on society will be difficult to measure scientifically, but measured clinically in productive years of life and a decreased reliance, for a time, on advanced levels of care (i.e. assisted living, nursing home, and in-home caregivers).
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023 Apr;19(4):1598-1695. doi: 10.1002/alz.13016. Epub 2023 Mar 14. PMID: 36918389.
- Citron M. Beta-secretase inhibition for the treatment of Alzheimer's disease--promise and challenge. Trends Pharmacol Sci. 2004 Feb;25(2):92-7. doi: 10.1016/j.tips.2003.12.004. PMID: 15102495.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297(5580):353-6. doi: 10.1126/science.1072994. Erratum in: Science 2002 Sep 27;297(5590):2209. PMID: 12130773.
- Behl C. Concerns about the amyloid cascade hypothesis and reappraisals. Springer eBooks. 2023 Jan 1; 167–83.
- Barage SH, Sonawane KD. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer's disease. Neuropeptides. 2015 Aug;52:1-18. doi: 10.1016/j.npep.2015.06.008. Epub 2015 Jul 2. PMID: 26149638.
- Kepp KP, Robakis NK, Høilund-Carlsen PF, Sensi SL, Vissel B. The amyloid cascade hypothesis: an updated critical review. Brain: A Journal of Neurology. 2023 May 15; 146(10): awad159. https://pubmed.ncbi.nlm.nih.gov/37183523/
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999 Jul 8;400(6740):173-7. doi: 10.1038/22124. PMID: 10408445.
- Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, Neal JW, Holmes C, Boche D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain. 2019 Jul 1;142(7):2113–26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6598630/
- Golde TE. Disease-Modifying Therapies for Alzheimer's Disease: More Questions than Answers. Neurotherapeutics. 2022 Jan;19(1):209-227. doi: 10.1007/s13311-022-01201-2. Epub 2022 Feb 28. PMID: 35229269; PMCID: PMC8885119.
- Chieh Lun Hsiao, Goro Katsuumi, Suda M, Shimizu I, Yoshida Y, Takaaki Furihata, Joki Y, Ryo Furuuchi, Hsiao Y, Liang J, Abe M, Tohru Minamino. Abstract P3004: Vaccination Targets Senescence-associated Glycoprotein Ameliorates Alzheimer’s Pathology and Cognitive Behavior in Mice. Circulation Research. 2023 Aug 4;133(Suppl_1).
- American Heart Association. Novel vaccine may hold key to prevent or reduce the impact of Alzheimer’s disease. American Heart Association. 2023. https://newsroom.heart.org/news/novel-vaccine-may-hold-key-to-prevent-or-reduce-the-impact-of-alzheimers-disease#:~:text=A%20novel%20vaccine%20that%20targets.
- Steenhuysen J. Researchers return to Alzheimer’s vaccines, buoyed by recent drug success. Reuters. 2023 Nov 20; https://www.reuters.com/business/healthcare-pharmaceuticals/researchers-return-alzheimers-vaccines-buoyed-by-recent-drug-success-2023-11-20/
- Taylor E. New Alzheimer’s drug, donanemab – what is it and how does it work?. Alzheimer’s Research UK. 2023. https://www.google.com/url?q=https://www.alzheimersresearchuk.org/blog/new-alzheimers-drug-donanemab-what-is-it-and-how-does-it-work/&sa=D&source=docs&ust=1703283008984186&usg=AOvVaw3vn7XOg8h4DwXx96s6wJos
- Abbott A. Could drugs prevent Alzheimer’s? These trials aim to find out. Nature. 2022 Mar 9; 603(7900):216–9. https://www.nature.com/articles/d41586-022-00651-0?utm_source=Nature+Briefing&utm_campaign=29ec12cca4-briefing-dy-20220309&utm_medium=email&utm_term=0_c9dfd39373-29ec12cca4-45021017#ref-CR5
- Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019 Feb;15(2):73-88. doi: 10.1038/s41582-018-0116-6. PMID: 30610216.
- Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, Suhy J, Forrestal F, Tian Y, Umans K, Wang G, Singhal P, Budd Haeberlein S, Smirnakis K. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022 Jan 1;79(1):13-21. doi: 10.1001/jamaneurol.2021.4161. PMID: 34807243; PMCID: PMC8609465.
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in early Alzheimer’s Disease. New England Journal of Medicine. 2022 Nov 29;388(1):9–21. https://www.nejm.org/doi/full/10.1056/NEJMoa2212948
- Warshawsky K. Aducanumab and Lecanemab: How are they different?. seniorsbluebook.com. 2023. https://seniorsbluebook.com/articles/aducanumab-and-lecanemab-how-are-they-different#:~:text=Aducanumab%20works%20by%20removing%20beta
- Iwatsubo T, Saido TC, Mann DM, Lee VM, Trojanowski JQ. Full-length amyloid-beta (1-42(43)) and amino-terminally modified and truncated amyloid-beta 42(43) deposit in diffuse plaques. Am J Pathol. 1996 Dec;149(6):1823-30. PMID: 8952519; PMCID: PMC1865366.
- Shcherbinin S, Evans CD, Lu M, Andersen SW, Pontecorvo MJ, Willis BA, Gueorguieva I, Hauck PM, Brooks DA, Mintun MA, Sims JR. Association of amyloid reduction after Donanemab treatment with tau pathology and clinical outcomes. JAMA Neurology. 2022 Oct 1; 79(10):1015.
- Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. doi: 10.1056/NEJMoa2100708. Epub 2021 Mar 13. PMID: 33720637.
- Yadollahikhales G, Rojas JC. Anti-Amyloid Immunotherapies for Alzheimer's Disease: A 2023 Clinical Update. Neurotherapeutics. 2023 Jul;20(4):914-931. doi: 10.1007/s13311-023-01405-0. Epub 2023 Jul 25. PMID: 37490245; PMCID: PMC10457266.
- Cummings J, Osse AML, Cammann D, Powell J, Chen J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer's Disease. BioDrugs. 2023 Nov 13. doi: 10.1007/s40259-023-00633-2. Epub ahead of print. PMID: 37955845.
- Biogen. A Phase 2, multicenter, randomized, parallel-group, double-blind, controlled study of aducanumab (BIIB037) in subjects with mild cognitive impairment due to Alzheimer’s disease or with mild Alzheimer’s disease dementia to evaluate the safety of continued dosing in subjects with asymptomatic amyloid-related imaging abnormalities. clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT03639987
- Biogen. A phase 3 multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of aducanumab (BIIB037) in subjects with early Alzheimer’s disease. clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT02477800
- Biogen. A phase 3 multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of Aducanumab (BIIB037) in subjects with early Alzheimer’s disease. clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT02484547
- Herring WL, Gould IG, Fillit H, Lindgren P, Forrestal F, Thompson R, Pemberton-Ross P. Predicted Lifetime Health Outcomes for Aducanumab in Patients with Early Alzheimer's Disease. Neurol Ther. 2021 Dec;10(2):919-940. doi: 10.1007/s40120-021-00273-0. Epub 2021 Aug 23. PMID: 34426940; PMCID: PMC8571451.
- Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, Lannfelt L, Bradley H, Rabe M, Koyama A, Reyderman L, Berry DA, Berry S, Gordon R, Kramer LD, Cummings JL. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021 Apr 17;13(1):80. doi: 10.1186/s13195-021-00813-8. Erratum in: Alzheimers Res Ther. 2022 May 21;14(1):70. PMID: 33865446; PMCID: PMC8053280.
- Shcherbinin S, Evans CD, Lu M, Andersen SW, Pontecorvo MJ, Willis BA, Gueorguieva I, Hauck PM, Brooks DA, Mintun MA, Sims JR. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes: the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurology. 2022 Oct 1;79(10):1015–24.
- Pontecorvo MJ, Lu M, Burnham SC, Schade AE, Dage JL, Shcherbinin S, Collins EC, Sims JR, Mintun MA. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022 Dec 1;79(12):1250-1259. doi: 10.1001/jamaneurol.2022.3392. PMID: 36251300; PMCID: PMC9577883.
- Eli Lilly and Company. Lilly’s Donanemab significantly slowed cognitive and functional decline in phase 3 study of early Alzheimer’s disease. Eli Lilly and Company. 2023 May 3; https://investor.lilly.com/news-releases/news-release-details/lillys-donanemab-significantly-slowed-cognitive-and-functional
- Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM; TRAILBLAZER-ALZ 2 Investigators. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023 Aug 8;330(6):512-527. doi: 10.1001/jama.2023.13239. PMID: 37459141; PMCID: PMC10352931.
- Eli Lilly and Company. U.S. Food and Drug Administration issues complete response letter for accelerated approval of Donanemab. Eli Lilly and Company. 2021; https://investor.lilly.com/news-releases/news-release-details/us-food-and-drug-administration-issues-complete-response-0
- Sohma Y. Medicinal Chemistry Focusing on Aggregation of Amyloid-β. Chem Pharm Bull (Tokyo). 2016;64(1):1-7. doi: 10.1248/cpb.c15-00742. PMID: 26726739.
- Ozawa S, Hori Y, Shimizu Y, Taniguchi A, Suzuki T, Wang W, Chiu YW, Koike R, Yokoshima S, Fukuyama T, Takatori S, Sohma Y, Kanai M, Tomita T. Photo-oxygenation by a biocompatible catalyst reduces amyloid-β levels in Alzheimer’s disease mice. Brain. 2021 Apr 14;144(6):1884–97.
- Sinha P, Barocas JA. Cost-effectiveness of aducanumab to prevent Alzheimer's disease progression at current list price. Alzheimers Dement (N Y). 2022 Mar 7;8(1):e12256. doi: 10.1002/trc2.12256. PMID: 35282659; PMCID: PMC8900580.
- Cummings JL, Goldman DP, Simmons-Stern NR, Ponton E. The costs of developing treatments for Alzheimer's disease: A retrospective exploration. Alzheimers Dement. 2022 Mar;18(3):469-477. doi: 10.1002/alz.12450. Epub 2021 Sep 28. PMID: 34581499; PMCID: PMC8940715.
- Lin G, Whittington MD, Wright A, Agboola F, Herron-Smith S, Pearson SD, Rind D. Lecanemab for early Alzheimer’s disease final evidence report prepared for. Institute for Clinical and Economic Review; 2023. https://icer.org/wp-content/uploads/2023/04/ICER_Alzheimers-Disease_Final-Report_For-Publication_04172023.pdf
- Castro DM, Dillon C, Machnicki G, Allegri RF. The economic cost of Alzheimer's disease: Family or public health burden? Dement Neuropsychol. 2010 Oct-Dec;4(4):262-267. doi: 10.1590/S1980-57642010DN40400003. PMID: 29213697; PMCID: PMC5619058.
- Asher S, Priefer R. Alzheimer's disease failed clinical trials. Life Sci. 2022 Oct 1;306:120861. doi: 10.1016/j.lfs.2022.120861. Epub 2022 Aug 4. PMID: 35932841.
- Bullain S, Doody R. What works and what does not work in Alzheimer's disease? From interventions on risk factors to anti-amyloid trials. J Neurochem. 2020 Sep;155(2):120-136. doi: 10.1111/jnc.15023. Epub 2020 May 9. PMID: 32277473.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
