Annals of Alzheimer's and Dementia Care
Beyond the Diagnosis: Identifying Major Risk Factors for Dementia in a Clinical Setting
Noor Ahmed Khosa1, Syed Muhammad Essa1*, Shahrukh Shoaib1, Sakina Gul1 and Noman Haq2
1Department of Neurology, Bolan Medical Complex Hospital, Quetta, Pakistan
2Faculty of Pharmacy and Health Sciences, University of Balochistan, Pakistan
Cite this as
Khosa NA, Essa SM, Shoaib S, Gul S, Haq N. Beyond the Diagnosis: Identifying Major Risk Factors for Dementia in a Clinical Setting. Ann Alzheimers Dement Care. 2025;9(1):001-010. Available from: 10.17352/aadc.000029Copyright
© 2025 Khosa NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Dementia is a serious health issue, and effective management requires an understanding of its risk factors. The purpose of this study was to assess dementia risk factors in patients from Bolan Medical Complex Hospital, Quetta.
Methods: From April 2021 to April 2024, a cross-sectional study was carried out with participants aged 18 and older who had been diagnosed with dementia using DSM-5 criteria. Demographic and risk factor-related data were collected through structured interviews, and cognitive status was assessed using the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA).
Results: The study included 178 patients, selected from a convenience sample. We found that two important independent risk factors for dementia were stroke (p < 0.001) and Wilson’s disease (p < 0.001). Significant correlations were observed between other dementia subtypes and stroke (OR = 0.339, 95% CI: 0.195 - 0.583) and Wilson’s Disease (OR = 0.424, 95% CI: 0.297 - 0.606). After adjusting for confounding factors, no additional variables were significantly associated with the risk of dementia, including age, gender, urbanization, socioeconomic status, diabetes, thyroid status, hypertension, family history, B12 deficiency, cardiovascular diseases, smoking, alcohol use, or physical activity.
Conclusion: It has been determined that stroke and Wilson’s disease are significant risk factors for dementia, especially the group of dementias other than Alzheimer’s and vascular dementia. According to these results, reducing the risk of dementia may benefit from focused screening and intervention for those with a history of stroke and Wilson’s disease. Additional longitudinal research is required to validate these correlations and investigate other risk factors.
Introduction
Dementia is a major public health issue, defined by a persistent deterioration in cognitive function that interferes with everyday activities and quality of life [1,2]. The growing number of elderly people worldwide is contributing to an alarming increase in the burden of dementia [3]. The World Health Organization (WHO) predicts that by 2050, there will be 152 million dementia sufferers worldwide, almost tripling from 55 million in 2020 [4-6]. This neurodegenerative disease affects not only patients but also imposes significant emotional and financial burdens on healthcare systems, caregivers, and families a great deal of emotional and financial burdens [7,8]. Even with dementia’s increasing prevalence, risk factors remain poorly understood, particularly in resource-limited settings like Balochistan, Pakistan [9].
Dementia is a syndrome associated with a variety of disorders, the most prevalent of which are Alzheimer’s disease, vascular dementia, Lewy body dementia, and frontotemporal dementia [10-12]. Although the specific cause of these disorders differs, it is widely known that both modifiable and non-modifiable risk factors play a role in their development [13]. Advanced age and genetic predisposition are key non-modifiable risk factors [14]. Nonetheless, lifestyle variables including physical inactivity and poor eating habits, as well as modifiable risk factors such as diabetes, hypertension, smoking, dyslipidemia, and cardiovascular diseases have garnered increasing attention in dementia preventive initiatives [15-17]. Understanding the distribution and impact of these risk factors is essential for developing region-specific preventative and treatment strategies.
Pakistan, the world’s fifth most populated country, is going through a demographic shift that is contributing to an aging population [18]. Despite this, dementia is frequently underdiagnosed and undertreated in areas with minimal resources because there is a lack of infrastructure and knowledge to manage the disease [19]. The largest yet least developed province in Pakistan, Balochistan, has more healthcare difficulties because of its isolation, socioeconomic limitations, and absence of specialized medical facilities [20]. Although the Bolan Medical Complex (BMC) Hospital in Quetta is a significant tertiary care facility that treats patients from all over the province, there is still a dearth of epidemiological data on neurological diseases like dementia. The current study seeks to better understand this expanding health concern on a local and global level by identifying the risk factors for dementia in patients who present to BMC Hospital, Quetta.
Several region-specific risk factors for dementia, such as cultural practices, socioeconomic conditions, and environmental exposures, have been discovered by previous studies [21,22]. Dementia risk has been associated with a number of characteristics in the South Asian environment, including low healthcare access, high rates of stroke and cardiovascular disease, and illiteracy [23,24]. However, there is a remarkable absence of data from Balochistan, a province with distinct ethnic, cultural, and environmental impacts. The absence of dementia research relevant to the region impedes the development of focused interventions, and the inadequate healthcare resources in Balochistan compound the difficulties experienced by patients and healthcare professionals.
This study aims not only to shed light on dementia prevalence in Balochistan, but it will also assist in developing public health initiatives targeted at the early detection, prevention, and treatment of dementia in this neglected area. Moreover, the research will enhance the wider comprehension of dementia risk factors in South Asia, a region where an exponential increase in the disease burden is anticipated in the upcoming decades. This research has the potential to help policymakers in Balochistan establish effective prevention initiatives, increase public awareness, and improve healthcare services for dementia patients by identifying important modifiable risk variables in the local community.
Methodology
Study design
This research utilized a hospital-based, cross-sectional observational methodology. The primary reason for selecting this methodology was its feasibility and efficacy in a resource-limited environment, like Balochistan, Pakistan. A hospital-based methodology facilitated direct access to a clinically diagnosed cohort of dementia patients, crucial for evaluating risk factors within a specified population. This design facilitated the collection of extensive demographic and clinical data at a single moment, offering a significant overview of the prevalence of diverse risk factors and their correlations with various dementia subtypes among the patient population at Bolan Medical Complex Hospital.
The primary objective was to identify and evaluate the risk factors associated with dementia in patients who visited the neurology Outpatient Department (OPD) from April 2021 to April 2024 over three years.
Study population
The study population consisted of individuals aged 18 and upwards who had been clinically diagnosed with dementia using recognized diagnostic criteria. Patients were recruited from the neurology outpatient department at Bolan Medical Complex Hospital in Quetta. Based on a convenience sample, 178 patients were included in the study.
Inclusion and exclusion criteria
Inclusion criteria:
Patients aged 18 years and above.
Patients with a clinical diagnosis of dementia.
Patients who provided informed consent to participate in the study.
Exclusion criteria:
Patients with reversible causes of cognitive impairment, such as metabolic disorders, depression.
Patients with severe psychiatric illnesses or other neurological disorders that could interfere with cognitive assessments.
Patients who were unwilling or unable to provide informed consent.
Criteria for diagnosis
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria were used to diagnose dementia [25]. According to the DSM-5, dementia also known as a major neurocognitive disorder is defined as evidence of a significant decline in cognitive function in comparison to one’s previous level of performance in one or more cognitive domains, including complex attention, executive function, language, learning and memory, perceptual-motor, or social cognition [26,27].
Data collection
Data were collected through face-to-face structured interviews. There were two sections on the questionnaire:
Demographic information included age, gender, ethnicity, and socioeconomic status were included in the demographic section.
Clinical and risk factor assessment: Data were acquired in this part regarding potential risk factors, such as cardiovascular disease, smoking, alcohol consumption, diabetes mellitus, hypertension, hyperlipidemia, physical inactivity, and dietary family history of Dementia.
Cognitive assessment
The Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) were used to evaluate each patient’s cognitive status [28]. These standardized tools were administered by neurologists.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). This particular version was selected due to its availability through institutional licensing and the research team’s familiarity with its interface and functionalities, ensuring consistency and efficiency in data processing. For the risk factors and demographic data, descriptive statistics were performed. Categorical variables were presented as frequencies and percentages, and continuous variables were given as means ± standard deviations (SD). Chi-square tests were applied for categorical data and independent t-tests were employed for continuous variables to evaluate the correlation between different risk factors and the occurrence of dementia. After accounting for relevant confounders, multivariate logistic regression analysis was used to determine independent dementia risk factors. For every risk factor, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Statistical significance was attained when the p - value was less than 0.05.
Results
The study evaluated several demographic, socioeconomic, and health-related risk factors to determine their association with dementia, particularly comparing patients diagnosed with Alzheimer’s disease to those with other kinds of dementia.
Demographic characteristics of the study population
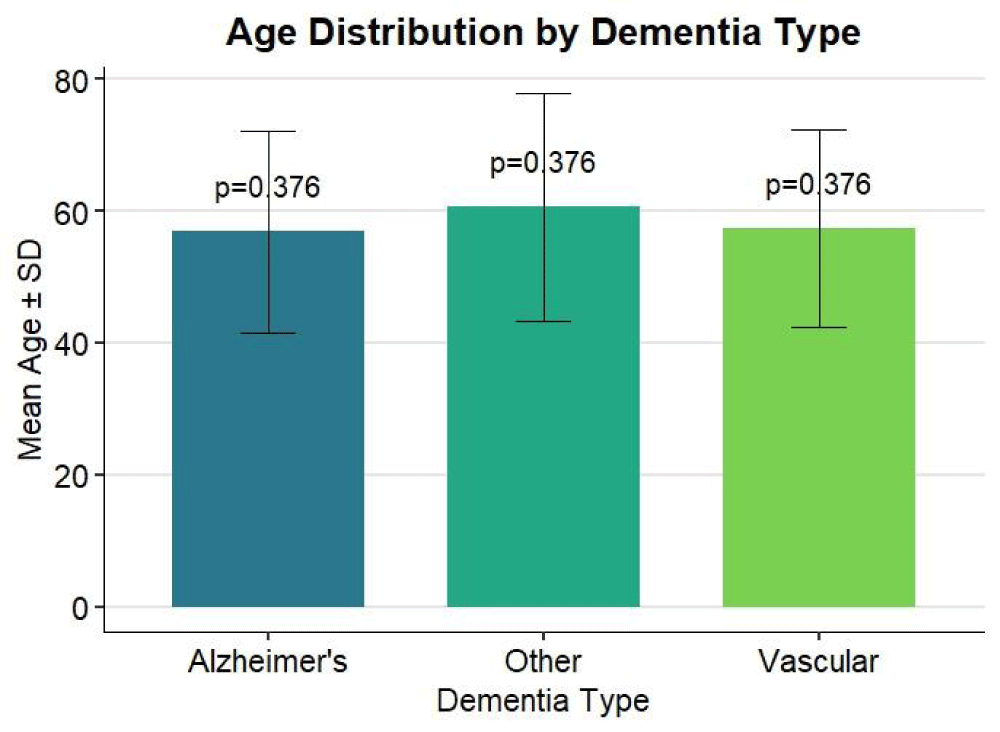
The mean age of participants varied slightly between dementia subtypes. A mean age of 56.97 ± 15.34 years was observed in the AD (Alzheimer’s disease) group, the standard deviation indicates some age fluctuation in this group, with the majority of the participants being middle-aged to older adults. The mean age of the vascular dementia (VD) group was 57.34 ± 14.99 years, which was slightly older than the overall group. With a slightly narrow standard deviation compared to the AD group and a minor rise in mean age, the VD group’s participants were generally older. With an average mean age of 60.60 ± 17.24 years, the other dementia (OD) group had the highest mean age, suggesting that, on average, this group included older people. The wider standard deviation reflects greater age variability within the group. The diversity might be explained by the fact that this category includes several less frequent types of dementia that might appear at different times of life. Despite these variances, the age differences between the groups were not statistically significant (p = 0.376), showing that age did not distinguish the dementia subtypes in the present study (Table 1).
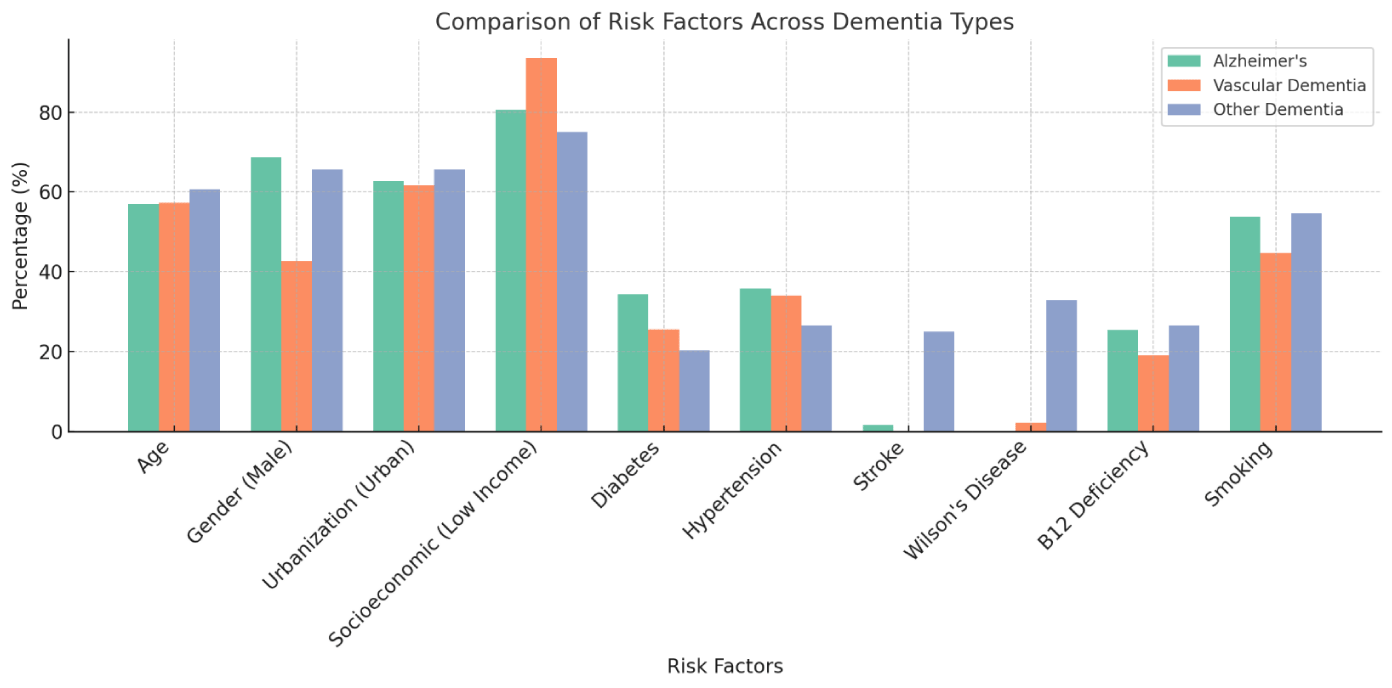
Significant differences in gender distribution were seen between the groups (p = 0.012). Males made up the majority in the AD group (68.7%), whereas females made up a larger proportion in the VD group (57.4%). With 65.6% males and 34.4% females, the gender distribution of the OD group was comparable to that of the AD group. These findings suggest potential gender-related differences in dementia prevalence (Figure 1).
The dementia subgroups were all consistent in terms of their place of residence, which was either urban or rural. Similar to 61.7% in the VD group and 65.6% in the OD group, 62.7% of the participants in the AD group were from urban regions. The fact that these differences were not statistically significant (p = 0.901) suggests that the type of dementia diagnosed was not significantly influenced by urbanization.
Across all groups, the majority had low socioeconomic levels; 80.6% of the AD group, 93.6% of the VD group, and 75.0% of the OD group were low-income. Income level did not significantly impact the likelihood of specific dementia subtype development in this cohort, as seen by the lack of statistically significant differences in socioeconomic status across the subtypes (p = 0.150).
Despite variations in ethnic background, there was no significant difference between the dementia subtypes (p = 0.098). There were 28.4% Baloch, 43.3% Pashtoon, 7.5% Persian, 11.9% Urdu-speaking, and 9.0% members of other ethnic groups in the AD group. With a few small differences, the distributions of the VD and OD groups were similar.
Risk factors for Dementia
The frequency of type 2 diabetes mellitus differed among the subtypes of dementia. Compared to 25.5% in the vascular dementia (VD) group and 20.3% in the other dementia (OD) group, 34.3% of individuals in the Alzheimer’s disease (AD) group had diabetes type2. Despite the fact that the AD group had a greater proportion of patients with diabetes, the differences were not statistically significant (p = 0.189). This implies that although diabetes is a prevalent co-occurring condition in dementia patients, it might not serve as a significant distinguishing feature among these subgroups (Figure 2)(Table 2).
The evaluation of thyroid disorders was conducted with consideration to normal thyroid function, hyperthyroidism, and hypothyroidism. 6.0% of patients in the AD group had hypothyroidism, 4.5% had hyperthyroidism, and 89.6% had normal thyroid function. In the VD group, there were 4.3% hypothyroidism cases, 2.1% hyperthyroidism cases, and 93.6% normal function cases. Comparably, 90.6% of the OD group had normal thyroid function, while 4.7% had both hyperthyroidism and hypothyroidism. Thyroid dysfunction was not significantly associated with specific dementia subtypes, as evidenced by the non-statistically significant changes in thyroid conditions across the groups (p = 0.942). Hypertension was detected in 35.8% of the AD group, 34.0% of the VD group, and 26.6% of the OD group. Despite being frequent, hypertension did not significantly differ across these groups, according to the distribution of hypertension across dementia subtypes, which was not statistically significant (p = 0.494). Nonetheless, its potential function as a general dementia risk factor is highlighted by the somewhat higher prevalence in the AD and VD groups.
There were significant differences across the dementia subtypes based on stroke incidence. A substantially higher 25.0% of participants in the OD group had a history of stroke than the 1.5% of participants in the AD group and none in the VD group. A history of stroke is strongly connected with other kinds of dementia and may be a distinguishing risk factor for non-vascular and non-Alzheimer’s dementia types. A highly significant correlation between dementia subtype and stroke incidence was identified by statistical analysis of these variations (p < 0.001). The data emphasize the significance of taking cerebrovascular events into account as a crucial component in the differential diagnosis and treatment of dementia, as the presence of stroke is a strong and significant risk factor for other dementias.
4.5% of individuals in the AD group, 2.1% in the VD group, and 6.3% in the OD group had a positive family history of dementia. Although family history is a known risk factor for dementia, the variations in family history among the subtypes were not statistically significant (p = 0.585), indicating that family history did not differ significantly among the dementia subtypes in this study.
Wilson’s disease afflicted 32.8% of individuals in the OD group, compared to none in the AD group and 2.1% in the VD group. This indicates that the OD group had a significantly higher prevalence of the disease. The significant difference (p < 0.001) indicates that Wilson’s disease is a crucial risk factor and this extremely significant p-value confirms that there is a meaningful link rather than a random variation in the prevalence of Wilson’s disease among the dementia subtypes. The results indicate that, in contrast to Alzheimer’s and vascular dementia, Wilson’s disease may be a significant risk factor that is particularly associated with other types of
B12 deficiency was noted in 26.6% of the OD group, 19.1% of the VD group, and 25.4% of the AD group. Despite the increased prevalence in the AD and OD groups, the differences were not statistically significant (p = 0.638).
The prevalence of cardiovascular diseases was 4.3% in the VD group, 6.3% in the OD group, and 4.5% in the AD group. Although noteworthy in the context of dementia, the slight variations in the prevalence of cardiovascular disease among the groups were not statistically significant (p = 0.861), indicating that cardiovascular diseases were not specifically linked to any particular dementia subtype in this study.
A smoking history was reported by 53.7% of patients with AD, 44.7% of VD patients, and 54.7% of OD patients. Despite being common, smoking did not substantially differ among the groups, according to the distribution of smoking patterns among the dementia subtypes (p = 0.531). Among the groups with dementia subtypes, 1.5% of AD, 2.1% of VD, and 1.6% of OD with statistical significance (p = 0.963) reported drinking, indicating minimal alcohol use across all groups.
We evaluated physical activity levels to determine their possible association with dementia risk. Regular physical activity was reported by 28.4% of participants in the AD group, 36.2% in the VD group, and 37.5% in the OD group. The variations in physical activity levels between the groups were not statistically significant (p = 0.497).
Correlation analysis
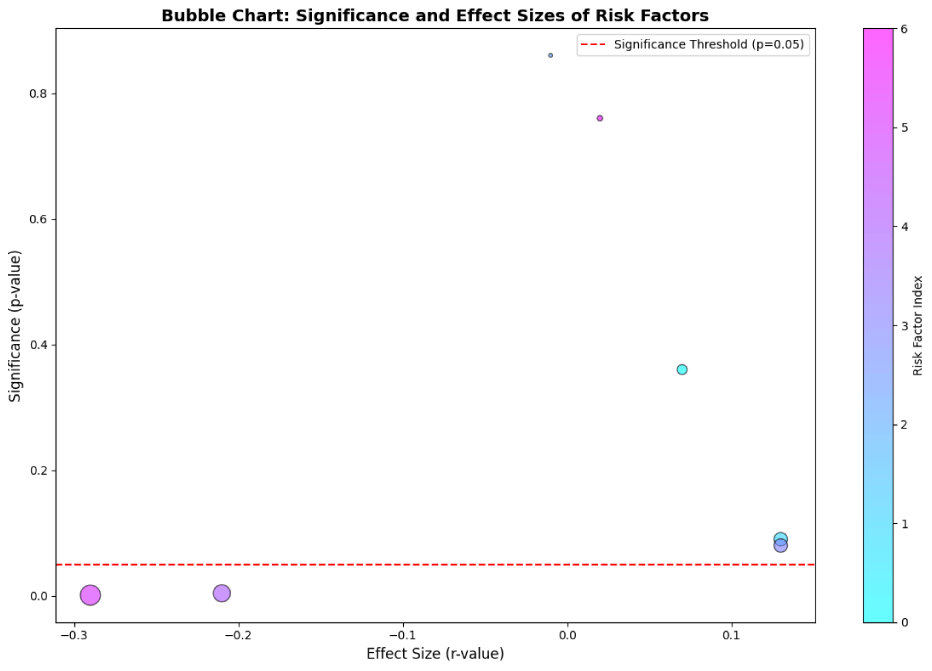
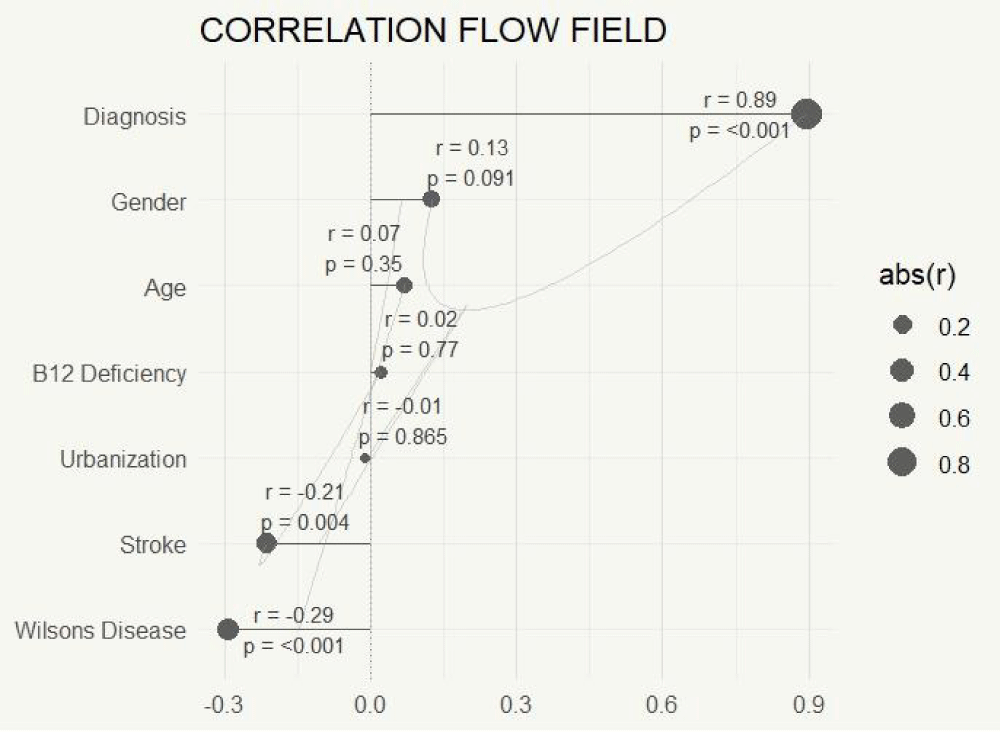
Correlation analyses were conducted to examine associations between different risk variables and dementia occurrence. There were low or non-significant associations between age, gender, urbanization, and socioeconomic position with dementia (p > 0.05). There was a noteworthy inverse relationship between dementia and stroke (r = -0.339, p < 0.001), indicating a lower stroke incidence among dementia groups compared to non-dementia groups.
Additionally, Wilson’s disease showed a strong inverse relationship with dementia (r = -0.424, p < 0.001), especially in the group with OD. The multivariate study provided additional confirmation of the relationship between dementia and stroke (Figures 3,4).
Multivariate logistic regression analysis
A multivariate logistic regression was used to identify independent risk factors for dementia while accounting for relevant confounders. Multivariate analysis identified stroke (OR = 0.339, 95% CI [0.195 – 0.583], p < 0.001) and Wilson’s disease (OR = 0.424, 95% CI [0.297 – 0.606], p < 0.001) as significant inverse predictors of dementia, indicating a lower likelihood of dementia in individuals with these conditions. No additional variables, encompassing demographics (gender, age, urbanization, socioeconomic status, ethnicity), clinical factors (diabetes, hypertension, thyroid dysfunction, B12 deficiency), or lifestyle factors (smoking, alcohol consumption, physical activity), exhibited statistically significant associations (all p ≥ 0.05). Odds ratios for non-significant factors were grouped around 1, with 95% confidence intervals encompassing 1, indicating the absence of meaningful effects. Notably, the initial bivariate significance of gender (p = 0.012) was not retained after multivariate adjustment (OR = 1.14, p = 0.435), indicating confounding factors. In summary, only stroke and Wilson’s disease independently forecast a reduction in dementia risk (Table 3).
Discussion
This cross-sectional study, conducted in a hospital setting, sought to identify and assess the risk factors linked to Alzheimer’s disease (AD), vascular dementia (VD), and other dementias (OD) in patients undergoing outpatient neurology care at Bolan Medical Complex Hospital in Quetta. A wide range of patients were included in the trial, which was conducted from April 2021 to April 2024, a duration of three years. The main conclusions showed that Wilson’s disease and stroke were important independent risk factors for dementia, especially other dementias (OD).
The ‘other dementias’ (OD) group, comprising a substantial segment of our cohort, signifies a diverse category that includes several atypical forms of dementia. Our analysis found Wilson’s disease as a notable independent risk factor within this cohort, highlighting its significance as a treatable cause of neurocognitive impairment, especially in younger people. In addition to Wilson’s disease, this category includes, but is not restricted to, Lewy body dementia, frontotemporal dementia, Huntington’s disease, Creutzfeldt-Jakob disease, Parkinson’s disease dementia, and dementias resulting from HIV infection, severe brain injury, or persistent substance misuse. Accurate identification of these disorders typically necessitates an extensive clinical assessment, encompassing a thorough neurological examination, targeted neuroimaging, and occasionally genetic or cerebrospinal fluid study, which may pose difficulties in specific clinical environments like Balochistan.
Unexpectedly, after adjusting for confounders, several commonly associated variables, including age, gender, urbanization, socioeconomic status, diabetes, thyroid status, hypertension, family history, B12 deficiency, cardiovascular diseases, smoking, alcohol use, and physical activity, did not show any significant associations with the risk of dementia.
It is critical to recognize the unique clinical concepts and etiologies associated with Early-Onset Dementia (EOD), which is commonly defined as dementia diagnosed before the age of 65. EOD often results from underlying causes such as genetic predisposition, specific neurological disorders, and rapidly advancing dementias, which may contrast with the more prevalent late-onset variants like Alzheimer’s disease and standard vascular dementia. Our study encompassed individuals aged 18 and older, with a mean age of the entire cohort ranging from 56.97 to 60.60 years across subtypes, indicating that the majority of our sample consisted of middle-aged to older persons. The notable correlation between Wilson’s disease and the ‘other dementias’ category is especially relevant, as Wilson’s disease is an established etiology of neurocognitive deficits that may present at a younger age. Our findings are consistent with Chen’s study, in which age is a primary risk factor for dementia, indicating the intrinsic susceptibility of the aging brain. However, Chen’s findings, which show a rapid increase in dementia frequency in people over 85, contradict with our lack of substantial age differences among subtypes. This implies that whereas aging generally predisposes people to dementia, other variables may influence subtype differentiation [29].
Our study’s gender distribution showed a female preponderance in VD and a male predominance in AD and OD, which supports Launer’s meta-analysis, which identified female sex as a significant risk factor for AD. Differences in cardiovascular risk profiles or hormonal changes during menopause may have an impact on this gender-related vulnerability, especially for vascular dementia in women [29].
In contrast with studies like Killin’s comprehensive review, which correlates environmental exposures like air pollution to an increased risk of dementia, especially in urban populations, our data show no discernible urban/rural residence effects [30]. Although our analysis did not identify statistically significant correlations between lifestyle, diet, and education and dementia risk, these factors continue to hold significance. This may result from data categorization, more pronounced influences from other risk factors, or the limitations of a cross-sectional study. Considering the diversity of our community, future research should collect more comprehensive data on diet, physical activity, and education to further the understanding of their influence on dementia risk, especially in Balochistan.
This study found a higher prevalence of diabetes in Alzheimer’s disease (AD) (34.3%) compared to vascular dementia (VD) (25.5%) and other dementias (20.3%), although the differences were not statistically significant. A meta-analysis by Cheng, et al. showed that diabetes significantly increases the risk for AD (RR: 1.46), VD (RR: 2.48), and all dementia types (RR: 1.51) [31]. Similarly, Kloppenborg, et al. in a systematic analysis of longitudinal studies, identified diabetes as a key contributor to dementia incidence, consistent with its multifaceted function in cognitive decline [32]. According to Albai, et al. diabetes duration and related complications, such as hyperglycemia and cardiovascular disease, are important risk factors for dementia progression, especially for Mild Cognitive Impairment (MCI) conversion [33]. Although Yu, et al. found that patients with diabetes had a greater incidence of AD than VD, this study’s lack of statistical significance could be due to sample size limits or population-specific variations [34]. In order to reduce the risk of dementia, these findings highlight the multifaceted role of diabetes in dementia pathogenesis, which is mediated by vascular injury, neurodegeneration, and metabolic dysregulation. They also emphasize the importance of early intervention and targeted prevention strategies for diabetes.
In this study, stroke emerged as a significant independent predictor of dementia risk, especially for other dementias (OD), where its frequency was significantly greater (25%) than that of vascular dementia (VD, 0%), and Alzheimer’s disease (AD, 1.5%). This research supports and adds to the body of literature by highlighting the crucial role that cerebrovascular events play in the pathophysiology of dementia and cognitive decline. There is substantial evidence linking dementia to stroke. According to McGrath, et al. stroke had a risk ratio (HR) of 3.61 (95% CI 2.21 – 5.92) at age 70, making it a powerful predictor of dementia [35]. Notably, our study found that stroke prevalence was unexpectedly higher in non-VD subtypes compared to vascular dementia, indicating that its effects may go beyond vascular dementia and impact non-traditional dementia subtypes through mechanisms such as neuroinflammation, neuronal death, or dysregulation of amyloid pathology. Similarly, Barba, et al. discovered that 30% of stroke patients developed dementia within three months of the stroke, regardless of type or site [36]. This challenges the conventional notion that vascular events solely underlie vascular dementia by this, which is consistent with our finding that stroke represents a significant risk factor across dementia subtypes. Reconciling long-held assumptions regarding the association between vascular dementia (VD) and cerebrovascular disease, our study found that the VD group did not have a history of stroke. This discrepancy may stem from our methodology, which primarily captured major cerebrovascular events while neglecting minor vascular brain damage, such as small vessel disease or microbleeds. Furthermore, several patients with vascular pathology may have been classified as having other dementias, with stroke being a notable contributing component (25%), indicating possible diagnostic ambiguity.
This study identified no significant variations in thyroid dysfunction among dementia subtypes, implying that thyroid disease has little impact on dementia risk in the study population. According to Wieland, et al. hypothyroidism is a substantial risk factor for dementia in people 65 and older, especially in those undergoing treatment (aOR 3.17, p = 0.043). These findings contrast with their results [37]. In a similar vein, Li-Yun Ma, et al.’s meta-analysis found that while hypothyroidism had no discernible impact, hyperthyroidism (RR = 1.14) and subclinical hyperthyroidism (RR = 1.56) were associated with an elevated risk of dementia [38]. Alšauskė, et al. highlighted the complex nature of these relationships, Highlighting heterogeneity among studies and cognitive outcome measures [39]. These discrepancies may be attributed to demographic variations, differing prevalence of thyroid disease, or methodological limitations, the prevalence of thyroid disease, or methodological constraints.
This study identified no significant variations in the prevalence of hypertension or vitamin B12 deficiency among dementia subtypes, indicating that these factors may not play a significant role in identifying dementia types in our population. However, these findings contradict the previous literature. Sharp, et al. found a link between hypertension and vascular dementia (VaD) in longitudinal (OR 1.59, CI 1.29 - 1.95, p < 0.0001) and cross-sectional studies (OR 4.84, CI 3.52 - 6.67, p < 0.00001), while Yen, et al. found a link between comorbid hypertension and type 2 diabetes and an increased risk of all-cause dementia and VaD (aHR 2.30, CI 1.71 - 3.13) [40,41]. Our results align with those of Siswanto, et al. that there is no substantial correlation between serum B12 levels and dementia [42]. O’Leary, et al. however, showed that sophisticated biomarkers, including holo transcobalamin and methylmalonic acid, showed correlations with cognitive deterioration [43].
This study underscores the multifactorial nature of dementia risk, highlighting the complex interplay between vascular, metabolic, and neurological factors. Although there were notable correlations between stroke and diabetes, further research is needed to fully understand the effects of hypertension, thyroid disorders, and vitamin B12 insufficiency, especially when considering population-specific factors. When compared to previous studies, our results emphasize the necessity for more thorough investigations employing reliable biomarkers and longitudinal designs in order to improve our comprehension of the genesis of dementia and promote specialized preventative measures.
Conclusion
This study analyzed the risk factors and demographic traits linked to different subtypes of dementia, focusing on thyroid dysfunction, stroke, type 2 diabetes, hypertension, and vitamin B12 deficiency. Our findings highlight the importance of stroke and Wilson disease in raising dementia risk, notably Alzheimer’s disease (AD) and other dementias (OD). Although few significant correlations were found between vitamin B12 deficiency, thyroid dysfunction, and hypertension, comparisons with previous research indicate that these conditions may still affect particular groups. These findings underline the necessity of early vascular and metabolic health management to reduce dementia risk and emphasize the need for further research, particularly in relation to advanced biomarkers and longitudinal research, to enhance preventive strategies and deepen our understanding of dementia pathogenesis.
Ethical considerations
The study followed the ethical criteria mentioned in the Declaration of Helsinki. The Post Graduate Medical Institute Quetta Institutional Review Board (IRB) granted ethical approval. All participants provided informed consent before being included in the study, ensuring their privacy and freedom to discontinue participation at any time.
- Barros D, Borges-Machado F, Silva-Fernandes A, Ribeiro O, Carvalho J. Do physical fitness and cognitive function mediate the relationship between basic activities of daily living and quality of life in older adults with dementia? Qual Life Res. 2024;33(4):917-926. Available from: https://link.springer.com/article/10.1007/s11136-023-03570-3
- Munawar K, Fadzil Z, Choudhry FR, Kausar R. Cognitive functioning, dependency, and quality of life among older adults. Act Adapt Aging. 2024;48(1):115-140. Available from: http://dx.doi.org/10.1080/01924788.2023.2193786
- Chen S, Cao Z, Nandi A, Counts N, Jiao L, Prettner K, et al. The global macroeconomic burden of Alzheimer's disease and other dementias: estimates and projections for 152 countries or territories. Lancet Glob Health. 2024;12(9):e1534-e1543. Available from: https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(24)00264-X/fulltext
- Jain M, Hogervorst E. The impact of the dementia epidemic. In: Design for Dementia. London: Routledge; 2024. p. 5-17.
- Shirai K, Iso H. Dementia. In: Social determinants of health in non-communicable diseases: Case studies from Japan. Tokyo: National Center for Global Health and Medicine. 2020;105-123.
- London O. Alzheimer's & Dementia: The Journal of the Alzheimer's Association.
- Duplantier SC, Williamson FA. Barriers and facilitators of health and well-being in informal caregivers of dementia patients: a qualitative study. Int J Environ Res Public Health. 2023;20(5):4328. Available from: https://www.mdpi.com/1660-4601/20/5/4328
- Knapp M, Wong G. Economics and dementia: Challenges and responses. Dementia. 2024;23(3):512-522. Available from: https://journals.sagepub.com/doi/10.1177/14713012231193141
- Balouch S, Zaidi A, Farina N, Willis R. Dementia awareness, beliefs and barriers among family caregivers in Pakistan. Dementia. 2021;20(3):899-918. Available from: https://journals.sagepub.com/doi/10.1177/1471301220915066
- Mehta RI, Schneider JA. Neuropathology of the common forms of dementia. Clin Geriatr Med. 2023;39(1):91-107. Available from: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0749069022000639
- Wei H, Masurkar AV, Razavian N. On gaps of clinical diagnosis of dementia subtypes: A study of Alzheimer’s disease and Lewy body disease. Front Aging Neurosci. 2023;15:1149036. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1149036
- Napoli E. Molecular, translational and clinical research on the two most common forms of neurodegenerative dementia: Alzheimer’s disease and dementia with Lewy bodies. Int J Mol Sci. 2023;24:7996. Available from: https://www.mdpi.com/1422-0067/24/9/7996
- Harrison Dening K. Dementia 1: types, risk factors, and recognising signs and symptoms. Nurs Times. 2023:2. Available from: https://www.nursingtimes.net/dementia/dementia-1-types-risk-factors-and-recognising-signs-and-symptoms-13-02-2023/
- Tippett LJ, Cawston EE, Morgan CA, Melzer TR, Brickell KL, Ilse C, et al. Dementia Prevention Research Clinic: a longitudinal study investigating factors influencing the development of Alzheimer’s disease in Aotearoa, New Zealand. J R Soc N Z. 2023;53(4):489-510. Available from: https://www.tandfonline.com/doi/full/10.1080/03036758.2022.2098780
- Jones A, Ali MU, Kenny M, Mayhew A, Mokashi V, He H, et al. Potentially modifiable risk factors for dementia and mild cognitive impairment: an umbrella review and meta-analysis. Dement Geriatr Cogn Disord. 2024;53(2):91-106. Available from: https://www.karger.com/Article/FullText/536643
- Carey A, Fossati S. Hypertension and hyperhomocysteinemia as modifiable risk factors for Alzheimer's disease and dementia: new evidence, potential therapeutic strategies, and biomarkers. Alzheimers Dement. 2023;19(2):671-695. Available from: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.12871
- Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)01296-0/abstract
- Mohan R. Fiscal challenges of population aging: the Asian experience. Reserve Bank India Bull. 2004 Oct. Available from: https://www.kansascityfed.org/documents/3398/PDF-Mohan2004.pdf
- Pathak KP. An overview of dementia. MedDocs Publ. 2019 [updated 2018]. Available from: https://meddocsonline.org/ebooks/an-overview-on-dementia/an-overview-on-dementia.pdf
- Haq SSNU. Essays on healthcare provision and utilization in Pakistan [dissertation]. University of Pau and the Pays de l'Adour. 2021. Available from: https://theses.hal.science/tel-04121868/
- Anstey KJ, Peters R, Zheng L, Barnes DE, Brayne C, Brodaty H, et al. Future directions for dementia risk reduction and prevention research: an international research network on dementia prevention consensus. J Alzheimers Dis. 2020;78(1):3-12. Available from: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-200674
- Klee M. Investigation of modifiable social and behavioural risk factors of cognitive decline and dementia. 2024.
- Hossain M, Crossland J, Stores R, Dewey A, Hakak Y. Awareness and understanding of dementia in South Asians: a synthesis of qualitative evidence. Dementia. 2020;19(5):1441-1473. Available from: https://journals.sagepub.com/doi/10.1177/1471301218800641
- Atcha M. Access to dementia diagnosis and support in a diverse South Asian community: a qualitative study [dissertation]. Lancaster University (United Kingdom); 2018. Available from: https://eprints.lancs.ac.uk/id/eprint/127056/1/2018AtchaPhD.pdf
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington (DC): Am Psychiatric Assoc. 2013;591-643.
- Luck T, Then FS, Schroeter ML, Witte V, Engel C, Loeffler M, et al. Prevalence of DSM-5 mild neurocognitive disorder in dementia-free older adults: results of the population-based LIFE-adult-study. Am J Geriatr Psychiatry. 2017;25(4):328-339. Available from: https://www.sciencedirect.com/science/article/pii/S1064748116304133
- Tay L, Lim WS, Chan M, Ali N, Mahanum S, Chew P, et al. New DSM-V neurocognitive disorders criteria and their impact on diagnostic classifications of mild cognitive impairment and dementia in a memory clinic setting. Am J Geriatr Psychiatry. 2015;23(8):768-779. Available from: https://www.sciencedirect.com/science/article/pii/S1064748115001080
- Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, et al. The Montreal Cognitive Assessment is superior to the Mini–Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr. 2012;24(11):1749-1755. Available from: https://www.cambridge.org/core/journals/international-psychogeriatrics/article/abs/montreal-cognitive-assessment-is-superior-to-the-minimental-state-examination-in-detecting-patients-at-higher-risk-of-dementia/64F8F6C1947B7DBD7F84787504105890
- Chen JH, Lin KP, Chen YC. Risk factors for dementia. J Formos Med Assoc. 2009;108(10):754-764. Available from: https://www.sciencedirect.com/science/article/pii/S0929664609604022
- Killin LO, Starr JM, Shiue IJ, Russ TC. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16:1-28. Available from: https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-016-0342-y
- Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484-491. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1445-5994.2012.02758.x
- Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585(1):97-108. Available from: https://www.sciencedirect.com/science/article/pii/S0014299908001789
- Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300-307. Available from: https://diabetesjournals.org/care/article/39/2/300/29442/Type-2-Diabetes-as-a-Risk-Factor-for-Dementia-in
- Yu JH, Han K, Park S, Cho H, Lee DY, Kim JW, et al. Incidence and risk factors for dementia in type 2 diabetes mellitus: a nationwide population-based study in Korea. Diabetes Metab J. 2020. Available from: https://e-dmj.org/journal/view.php?number=2503
- McGrath ER, Beiser AS, O'Donnell A, Himali JJ, Pase MP, Satizabal CL, et al. Determining vascular risk factors for dementia and dementia risk prediction across mid-to later life: the Framingham Heart Study. Neurology. 2022;99(2):e142-e153. Available from: https://n.neurology.org/content/99/2/e142
- Barba R, Martínez-Espinosa S, Rodríguez-García E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000;31(7):1494-1501. Available from: https://www.ahajournals.org/doi/10.1161/01.str.31.7.1494
- Wieland DR, Wieland JR, Wang H, Chen YH, Lin CH, Wang JJ, et al. Thyroid disorders and dementia risk: a nationwide population-based case-control study. Neurology. 2022;99(7):e679-e687. Available from: https://n.neurology.org/content/99/7/e679
- Ma LY, Zhao B, Ou YN, Zhang DD, Li QY, Tan L. Association of thyroid disease with risks of dementia and cognitive impairment: a meta-analysis and systematic review. Front Aging Neurosci. 2023;15:1137584. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1137584
- Alšauskė SV, Liseckienė I, Verkauskienė R. The association of thyroid disease with risk of dementia and cognitive impairment: a systematic review. Medicina. 2024;60(12):1917. Available from: https://www.mdpi.com/1010-660X/60/12/1917
- Sharp SI, Aarsland D, Day S, Sønnesyn H. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26(7):661-669. Available from: https://onlinelibrary.wiley.com/doi/10.1002/gps.2572
- Yen FS, Wei JC, Yip HT, Hwu CM, Hsu CC. Diabetes, hypertension, and the risk of dementia. J Alzheimers Dis. 2022;89(1):323-333. Available from: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-220207
- Siswanto O, Smeall K, Watson T, Donnelly-Vanderloo M, O'Connor C, Foley N, et al. Examining the association between vitamin B12 deficiency and dementia in high-risk hospitalized patients. J Nutr Health Aging. 2015;19:1003-1008. Available from: https://link.springer.com/article/10.1007/s12603-015-0660-3
- O'Leary F, Allman-Farinelli M, Samman S. Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr. 2012;108(11):1948-1961. Available from: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/vitamin-b12-status-cognitive-decline-and-dementia-a-systematic-review-of-prospective-cohort-studies/3DA69E726D3A3BECEBC897C36ED3C5A8

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
