Annals of Alzheimer's and Dementia Care
Recent Developments on Alzheimer’s Intervention: A Mini Review
Aina Oluwafemi S1* and Ojini Kelechi I2
1Biological Sciences Department, Trinity University, Lagos, Nigeria
2Medical Laboratory Science Department, Trinity University, Lagos, Nigeria
Cite this as
Aina Oluwafemi S, Ojini Kelechi I. Recent Developments on Alzheimer’s Intervention: A Mini Review. Ann Alzheimers Dement Care. 2025;9(1):011-016. Available from: 10.17352/aadc.000030Copyright
© 2025 Aina Oluwafemi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by amyloid-beta plaque deposition, tau protein tangles, neuronal loss, and cerebral atrophy, leading to cognitive decline and memory impairment. Traditionally, treatments have primarily addressed symptoms without targeting the disease’s underlying pathology. However, recent advances have spurred the development of disease-modifying therapies aimed at halting or reversing AD progression. This review highlights key therapeutic strategies, including targeting amyloid-beta and tau protein pathology, natural compounds like curcumin and resveratrol, and anti-inflammatory or antioxidant approaches. Emerging evidence suggests that bacterial infections, such as Porphyromonas gingivalis associated with periodontal disease, may contribute to AD pathogenesis through mechanisms involving neuroinflammation and amyloid production. Novel therapeutic approaches leveraging computational drug discovery and combinatorial treatments offer promising avenues for combating this multifaceted disease. By integrating insights from both traditional and emerging research, this review underscores the importance of a multi-modal strategy in addressing Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD) represents a significant global challenge, particularly among aging populations where its incidence continues to rise. It is classically defined by the accumulation of extracellular amyloid-beta plaques, intracellular neurofibrillary tangles composed of hyperphosphorylated tau, neuronal degeneration, and cerebral atrophy. The biological definition, as proposed by Dubois, et al. [1] emphasizes amyloid and tau pathology without explicitly including neuroinflammation, although inflammation is frequently observed in many AD cases. Traditionally, treatments for Alzheimer’s have centered on symptom relief rather than addressing the underlying pathology. However, recent advancements have redirected focus toward disease-modifying therapies aimed at slowing or halting the progression of the disease [2].
In the last five years, considerable progress has been made in elucidating the molecular and cellular mechanisms underlying AD. Researchers have identified critical targets, such as amyloid-beta and tau proteins, that play essential roles in the disease’s pathogenesis [3,4]. Furthermore, advancements in computational technologies, like high-throughput screening, molecular docking, and in-silico drug discovery, have expedited the identification and optimization of potential therapeutic agents [4].
This review emphasizes significant chemical compounds developed for Alzheimer’s intervention over the past seven years, encompassing both synthetic and natural options, while exploring their mechanisms, effectiveness, and future prospects in the fight against Alzheimer’s disease.
Targeting amyloid-beta pathology
Amyloid-beta pathology is a key factor in the development of Alzheimer’s disease (AD). Therapeutic strategies targeting this pathology include BACE1 inhibitors, which inhibit the production of amyloid-beta, and amyloid aggregation inhibitors like TRIAD 12, which prevent the formation of amyloid plaques. Despite varying clinical outcomes, these approaches are essential for efforts to slow AD progression.
Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) plays a vital role in producing amyloid-beta peptides, which aggregate to form plaques in the brains of individuals with AD. Targeting BACE1 has become a central focus in Alzheimer’s research. BACE1 inhibitors, such as verubecestat, have been developed and evaluated in clinical trials for their ability to reduce amyloid-beta levels. A study conducted by Resende, et al. [5] assessed the efficacy of verubecestat in patients with mild-to-moderate AD. Although the drug effectively lowered amyloid-beta levels, it did not result in significant cognitive improvements. These findings have raised concerns regarding the efficacy of amyloid-beta-targeting therapies as standalone treatments. Recent studies are now investigating combination therapies, where BACE1 inhibitors are used alongside agents that target other mechanisms related to AD, such as tau protein pathology. This multi-targeted strategy could provide a more comprehensive approach to addressing the complex pathology of Alzheimer’s disease.
In addition to inhibiting BACE1, another strategy involves directly targeting the aggregation of amyloid-beta into plaques. New amyloid-beta aggregation inhibitors, such as TRIAD 12, have emerged as promising options. TRIAD 12 is a small-molecule inhibitor that effectively reduces amyloid-beta fibrillization, demonstrating significant efficacy in decreasing plaque formation and enhancing cognitive function in preclinical models [6]. A notable advancement in amyloid-targeting therapies occurred with the FDA approval of aducanumab in 2021. Aducanumab is a monoclonal antibody designed to target amyloid plaques and was celebrated as a groundbreaking treatment. However, its approval was met with controversy due to inconsistent results from clinical trials. While aducanumab successfully lowered amyloid plaque levels, its cognitive benefits varied significantly among patient populations [4]. This underscores the complexity of amyloid-beta as a target and emphasizes the need for a multi-modal approach that not only addresses plaque formation but also considers other pathological aspects of AD. Verubecestat, although promising in mechanism, led to increased neuropsychiatric side effects in Phase III trials (EPOCH study), prompting early termination [5]. Lecanemab, an amyloid protofibril-targeting antibody, showed significant reduction in cognitive decline by 27% in early AD patients in a global Phase III CLARITY-AD trial [4].
Modulating tau protein pathology
Tau protein abnormalities are another defining characteristic of Alzheimer’s disease, with hyperphosphorylated tau forming neurofibrillary tangles that contribute to neurodegeneration. Therefore, targeting tau protein pathology has become a crucial focus for therapeutic development.
It has been suggested that SF, a CDK5 inhibitor, reduces the expression of CDK5 as well as the kinase activity of CDK5/p25 both in vitro and in vivo models of AD [7]. While these inhibitors show promise, clinical development is ongoing, with further trials needed to evaluate their safety and efficacy in human patients.
Microtubule Stabilizers are another strategy for modulating tau pathology centers on stabilizing tau-microtubule interactions. Normally, tau binds to and stabilizes microtubules; however, hyperphosphorylated tau loses this capability, leading to neuronal dysfunction. Agents that stabilize microtubules, such as epothilones, are being investigated for their potential to restore this function. Epothilone D has shown efficacy in stabilizing neuronal microtubules and preventing tau-related neurodegeneration in preclinical studies [8]. Current clinical trials are assessing whether these compounds can slow the progression of AD in patients. In preclinical mouse models, Patupilone (Epothilone D) significantly reduced axonal swelling and tau pathology at low doses, with improved spatial memory.
Natural compounds as alzheimer’s interventions
Natural compounds have long served as a valuable source of therapeutic agents in medicine, and their potential for treating Alzheimer’s disease (AD) is no exception. Numerous naturally occurring substances have been identified that can influence the key pathways involved in AD, particularly those related to amyloid-beta and tau pathology. Curcumin, a bioactive compound found in turmeric, has garnered attention for its antioxidant, anti-inflammatory, and amyloid-inhibitory effects. Despite its promising potential, the therapeutic application of curcumin has been hindered by its low bioavailability. Recent studies have focused on creating curcumin analogs and developing nanoparticle-based delivery systems to enhance its bioavailability and effectiveness. Wang, et al. [2] demonstrated that curcumin-loaded nanoparticles significantly improved cognitive function and reduced amyloid burden in mouse models of AD. These results indicate that with enhanced formulations, curcumin could have a significant role in future interventions for Alzheimer’s disease.
Resveratrol, a polyphenolic compound found in grapes and berries, has shown considerable promise in modulating neuroinflammation and promoting the clearance of amyloid-beta. It acts by activating sirtuin 1 (SIRT1), a protein that plays a critical role in cellular health and longevity. A study by Yan, et al. [9] found that resveratrol not only lowered amyloid-beta levels but also improved synaptic plasticity and cognitive function in animal models. Ongoing clinical trials aim to evaluate the potential of resveratrol in human patients, with particular emphasis on its long-term safety and efficacy.
Earlier reports have shown phase II clinical trial (NCT01504854) of resveratrol with promising inhibitory potential but thereafter, concerns were raised due to brain volume reduction, indicating possible blood-brain barrier effects [10]. Berberine, an isoquinoline alkaloid, also reduced tau hyperphosphorylation via PI3K/Akt/GSK3β signaling and enhanced autophagic clearance of amyloid in APP/PS1 mice [11]. Ginkgo biloba extract EGb 761 were found to improve symptoms in mild AD but showed limited long-term efficacy in large trials like GEM. Green tea catechin demonstrated reduction of Aβ-induced toxicity and aggregation in vitro and in AD transgenic mice [12]. Mediterranean diet has also been associated with reduced AD risk and slower cognitive decline, likely due to its richness in polyphenols and omega-3s [13].
Anti-inflammatory and antioxidant approaches
Chronic neuroinflammation and oxidative stress are well-recognized factors contributing to the pathogenesis of Alzheimer’s disease (AD). As a result, there is increasing interest in developing anti-inflammatory and antioxidant therapies for the condition. While non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen have been explored for their potential to alleviate neuroinflammation in AD, clinical trials have not consistently demonstrated cognitive benefits. However, recent research indicates that targeting specific inflammatory pathways may yield more favorable outcomes. For example, inhibiting the NLRP3 inflammasome, a major driver of neuroinflammation, has been shown to reduce amyloid-beta pathology and enhance cognitive function in AD models [14].
Oxidative stress is a critical factor in the early development of Alzheimer’s disease. Antioxidants like alpha-lipoic acid and N-acetylcysteine (NAC) have proven effective in mitigating oxidative damage in preclinical models of AD [15]. Recent studies suggest that combining antioxidants with other therapeutic strategies could offer more comprehensive neuroprotection. F /or instance, research by Dos, et al. [16] demonstrated that pairing alpha-lipoic acid with a tau aggregation inhibitor improved cognitive function in animal models, highlighting the potential of combinatorial antioxidant therapies. The selective COX-2 inhibitor celecoxib reduced neuroinflammatory markers in AD mouse models without the gastrointestinal side effects typical of traditional NSAIDs [15].
Cholinergic and glutamatergic modulation
Alzheimer’s disease is marked by significant dysfunction in both the cholinergic and glutamatergic systems. The cholinergic system, essential for memory and cognitive functions, is particularly compromised in AD, leading to the development of cholinesterase inhibitors such as donepezil and rivastigmine. Likewise, the glutamatergic system, especially the NMDA receptor, plays a crucial role in synaptic plasticity, with its overactivation contributing to excitotoxicity in the context of AD. Memantine, an NMDA receptor antagonist, is currently employed to mitigate excitotoxicity in patients with AD. However, recent advancements have focused on developing more selective NMDA receptor modulators that target specific subunits associated with synaptic plasticity and neuroprotection. Galantamine also acts as an allosteric modulator of nicotinic receptors, providing dual cholinergic and neuroprotective actions. These next-generation NMDA modulators have shown promise in preclinical studies, where they have been found to improve cognitive function and reduce neurotoxicity [17].
In-silico approaches and drug discovery
Molecular docking and virtual screening have been extensively utilized to discover novel inhibitors of amyloid-beta and tau aggregation. For example, a recent study by Pasieka, et al. [18] identified several phenylpiperazine derivatives as potent inhibitors of amyloid-beta aggregation and tau hyperphosphorylation. These compounds exhibited high binding affinity for both amyloid-beta and tau, resulting in neuroprotective effects in preclinical models.
Quantum mechanics/molecular mechanics (QM/MM) simulations provide a comprehensive understanding of how small molecules interact with their targets. This hybrid computational technique has been employed to examine the interactions between potential AD drugs and proteins like tau. Baggett and Nath [19] expanded the toolkit of protein aggregation inhibitors into new areas of chemical space and demonstrated the feasibility of ligand discovery strategy.
Expanding therapeutic hypotheses in alzheimer’s disease
While amyloid-beta-targeted interventions have dominated AD drug development, current research supports over 100 therapeutic hypotheses beyond the Aβ cascade. These include mechanisms involving inflammation, vascular dysfunction, microRNAs, and other comorbid dementias. Broadening the focus of AD interventions is essential to developing truly disease-modifying therapies.
General therapeutic perspectives
The amyloid hypothesis, though foundational, may be insufficient alone to explain AD progression. Min, et al. [20] emphasized the necessity to consider diverse molecular and cellular pathways in AD, such as mitochondrial dysfunction, synaptic failure, metal dyshomeostasis, and impaired proteostasis, arguing for combinatorial and multitargeted strategies for treatment. Similarly, Athar, et al. [21] reviewed molecular genetics of AD and suggested multi-pronged approaches involving genomics, transcriptomics, and proteomics to identify reliable biomarkers and novel therapeutic targets. Boxer, et al. [22] provided new mechanistic insights from single-cell analysis that reveal the cellular heterogeneity underlying AD pathophysiology.
Neuro-inflammation
Inflammation is increasingly recognized as a core contributor to AD, extending beyond a secondary response to plaque accumulation. Liang, et al. [23] demonstrated that the NLRP3 inflammasome, microglial activation, and cytokine signaling pathways play a central role in neurodegeneration, positioning anti-inflammatory agents and immunomodulators as potential therapeutic options.
MicroRNA dysregulation
MicroRNAs (miRNAs) regulate gene expression and are involved in neuronal survival, plasticity, and inflammation. Li et al. [24] explored the role of specific miRNAs in AD progression, focusing on their impact on β-amyloid (Aβ) peptide accumulation, intracellular aggregation of hyperphosphorylated tau proteins, mitochondrial dysfunction, neuroinflammation, oxidative stress, and the expression of the APOE4 gene. Their insights contributed to understanding AD’s pathology, offering new avenues for identifying diagnostic markers and developing novel therapeutic targets.
Vascular contributions
Cerebrovascular dysfunction significantly exacerbates AD pathology. Sery, et al. [25] reviewed the role of vascular endothelial function and cerebral perfusion in neurodegeneration and noted that AD may result from a combination of amyloid and vascular insults, reinforcing the concept of “vascular cognitive impairment” as an overlapping pathology.
Other ancient dementia-related pathologies
Recent findings suggest that pathologies historically associated with other forms of dementia—such as argyrophilic grain disease, hippocampal sclerosis, and TDP-43 proteinopathy—may also intersect with AD. Metah, et al. [26] examined the therapeutic potential of targeting these overlapping pathologies, advocating for stratified treatment strategies that consider co-pathologies in elderly patients with cognitive decline.
Microbiota and the gut-brain axis
The interplay between the gut-brain axis and Alzheimer’s disease (AD) has drawn increasing attention. Evidence suggests that gut dysbiosis contributes to AD pathogenesis by promoting neuroinflammation and amyloid-beta accumulation. Microbial metabolites, such as short-chain fatty acids (SCFAs), can cross the blood-brain barrier, influencing brain health. Modulating gut microbiota through probiotics or prebiotics is emerging as a potential therapeutic approach to reduce neuroinflammation and slow AD progression [27].
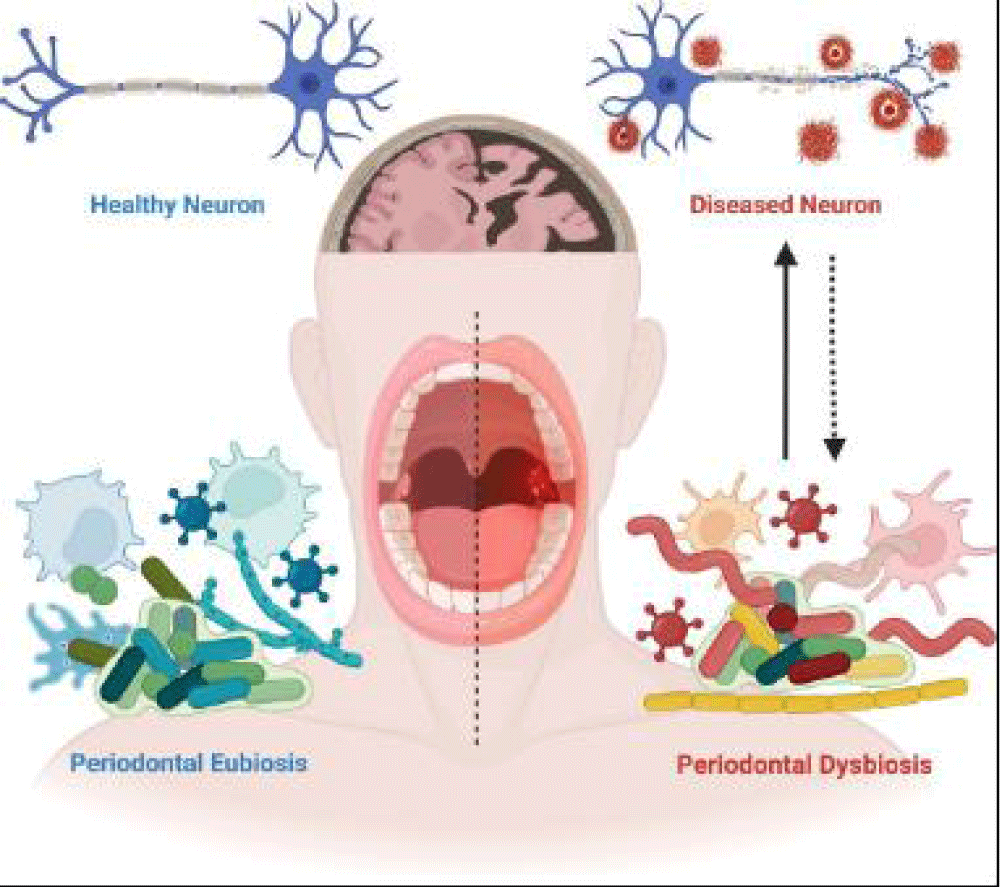
Periodontitis, a chronic inflammatory condition affecting the gums and surrounding structures, is linked to systemic health issues, including AD. The disease, caused by colonization of gram-negative bacteria like Porphyromonas gingivalis and other periodontal pathogens (Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum), is characterized by tissue destruction. Virulence factors such as gingipains and outer membrane vesicles from P. gingivalis can cross biological barriers, including the blood-brain barrier, and contribute to amyloid-beta pathology and neuroinflammation [28,29].
P. gingivalis DNA, lipopolysaccharides, and gingipains have been identified in brain tissues of AD patients, along with antibodies against multiple periodontal pathogens (T. denticola, A. actinomycetemcomitans, F. nucleatum, P. intermedia). This suggests a significant role of periodontal infections in AD progression [30,31]. Additionally, Treponema species have been found in the trigeminal ganglia and brain tissue, indicating neural pathways as a route for infection [32]. Clinical studies and systematic reviews support a bidirectional relationship between periodontal disease (PD) and AD. While PD increases the risk of developing dementia, cognitive decline in AD patients worsens oral hygiene, exacerbating PD [33,34]. Elevated cytokines, amyloid-beta peptides, tau hyperphosphorylation, hippocampal microgliosis, and neuronal death have been observed in both animal models and humans with periodontal infections. These findings highlight the role of systemic inflammation and brain infections in AD pathogenesis. Figure 1 illustrates the bidirectionality of PD and AD, where periodontal pathogens and their virulence factors influence brain pathology, while cognitive decline leads to poor oral hygiene and increased PD severity [35]. Addressing PD may offer a preventative strategy to mitigate the global dementia epidemic, with significant public health implications [34].
Challenges and future directions
One promising strategy to tackle the multifaceted nature of AD is the development of combination therapies. For instance, pairing amyloid-beta inhibitors with drugs targeting tau has shown additive or synergistic effects in animal studies. Similarly, combining antioxidants with anti-inflammatory agents offers broader neuroprotection. Ongoing clinical trials are currently investigating the efficacy of these combination therapies, with the hope of achieving more comprehensive disease-modifying results. A significant challenge in AD drug development is the effective delivery of therapeutic compounds to the brain. The Blood-Brain Barrier (BBB) poses a major obstacle in this regard. However, recent advances in nanotechnology have opened new avenues for improving the bioavailability and brain penetration of drugs. Lipid-based nanoparticles and polymeric nanocarriers have shown promise in delivering compounds like curcumin and resveratrol across the BBB, with encouraging results in preclinical studies [17].
Future research should focus on customizing interventions based on an individual’s genetic profile to maximize therapeutic efficacy. By leveraging a combination of imaging, genetic, novel ultrasensitive immunoassays, mass spectrometry methods, metabolomics, and exosomes, significant strides towards personalized healthcare for individuals with AD could be made [36].
Conclusion
Alzheimer’s disease remains one of the biggest challenges in healthcare today, largely because of how complex and varied its causes are. But there’s hope on the horizon. Natural compounds and modern computer-based drug discovery are opening exciting new possibilities for better treatments. Interestingly, recent research suggests that bacterial infections could play a role in the development of Alzheimer’s, pointing to the importance of considering microbes when designing therapies. For example, infections with Porphyromonas gingivalis have been linked to brain inflammation and faster buildup of amyloid plaques, thereby offering fresh ideas for preventing or slowing the disease.
Looking ahead, combining these different treatment strategies could make a real difference in reducing the toll Alzheimer’s takes on patients and families. At the same time, simple lifestyle changes, such as following the MIND diet and staying physically active with regular aerobic exercise, have shown promise in slowing memory decline and supporting brain health.
- Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, et al. Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484–96. Available from: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(21)00066-1/abstract
- Wang H, Sun M, Li W, Liu X, Zhu M, Qin H. Biomarkers associated with the pathogenesis of Alzheimer’s disease. Front Cell Neurosci. 2023;17:1279046. Available from: https://doi.org/10.3389/fncel.2023.1279046
- Bagga S, Kumar M. Current status of Alzheimer’s disease and pathological mechanisms investigating the therapeutic molecular targets. Curr Mol Med. 2023;23(6):492–508. Available from: https://doi.org/10.2174/1566524022666220404112843
- van Dyck CH. Anti-amyloid-beta monoclonal antibodies for Alzheimer's disease: Pitfalls and promise. Biol Psychiatry. 2022;91(2):134–46. Available from: https://doi.org/10.1016/j.biopsych.2017.08.010
- Resende R, Ferreira-Marques M, Moreira P, Coimbra JR, Baptista SJ, Isidoro C, et al. New BACE1 chimeric peptide inhibitors selectively prevent AβPP-β cleavage decreasing amyloid-β production and accumulation in Alzheimer’s disease models. J Alzheimers Dis. 2020;76(4):1317–37. Available from: https://doi.org/10.3233/JAD-200381
- Ma KS, Hasturk H, Carreras I, Dedeoglu A, Veeravalli JJ, Huang JY, Kantarci A, Wei JC. Dementia and the risk of periodontitis: A population-based cohort study. J Dent Res. 2022;101:270–7. Available from: https://doi.org/10.1177/00220345211037220
- Yang W, Xu QQ, Yuan Q, Xian YF, Lin ZX. Sulforaphene, a CDK5 Inhibitor, attenuates cognitive deficits in a transgenic mouse model of Alzheimer’s disease via reducing Aβ Deposition, tau hyperphosphorylation and synaptic dysfunction. Int Immunopharmacol. 2023;114:109504. Available from: https://doi.org/10.1016/j.intimp.2022.109504
- Wisniewski T, Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85(6):1162–76. Available from: https://www.cell.com/neuron/fulltext/S0896-6273(14)01173-8
- Yan Y, Yang H, Xie Y, Ding Y, Kong D, Yu H. Research progress on Alzheimer's disease and resveratrol. Neurochem Res. 2020;45:989–1006. Available from: https://link.springer.com/article/10.1007/s11064-020-03007-0
- Turner RS, Thomas RG, Craft S, Van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–91. Available from: https://doi.org/10.1212/WNL.0000000000002035
- Zhang W, Huai Y, Miao Z, Qian A, Wang Y. Systems pharmacology for investigation of the mechanisms of action of traditional Chinese medicine in drug discovery. Front Pharmacol. 2019;10:743. Available from: https://doi.org/10.3389/fphar.2019.00743
- Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MB. Green tea catechins as brain‐permeable, natural iron chelators‐antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res. 2006;50(2):229–34. Available from: https://doi.org/10.1002/mnfr.200500156
- Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–25. Available from: https://doi.org//10.1001/archneurol.2008.536
- Liang T, Zhang Y, Wu S, Chen Q, Wang L. The role of NLRP3 inflammasome in Alzheimer’s disease and potential therapeutic targets. Front Pharmacol. 2022;13:845185. Available from: https://doi.org/10.3389/fphar.2022.845185
- Pritam P, Deka R, Bhardwaj A, Srivastava R, Kumar D, Jha AK, et al. Antioxidants in Alzheimer’s disease: Current therapeutic significance and future prospects. Biology (Basel). 2022;11(2):212. Available from: https://doi.org/10.3390/biology11020212
- Dos Santos SM, Romeiro CFR, Rodrigues CA, Cerqueira ARL, Monteiro MC. Mitochondrial Dysfunction and Alpha‐Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease? Oxid Med Cell Longev. 2019;2019(1):8409329. Available from: https://doi.org/10.1155/2019/8409329
- Liu J, Chang L, Song Y, Li H, Wu Y. The role of NMDA receptors in Alzheimer’s disease. Front Neurosci. 2019;13:43. Available from: https://doi.org/10.3389/fnins.2019.00043
- Pasieka A, Panek D, Szałaj N, Espargaró A, Więckowska A, Malawska B, et al. Dual inhibitors of amyloid-β and tau aggregation with amyloid-β disaggregating properties: extended in cellulo, in silico, and kinetic studies of multifunctional anti-Alzheimer’s agents. ACS Chem Neurosci. 2021;12(11):2057–68. Available from: https://pubs.acs.org/doi/full/10.1021/acschemneuro.1c00235
- Baggett DW, Nath A. The rational discovery of a tau aggregation inhibitor. Biochemistry. 2018;57(42):6099–107. Available from: https://pubs.acs.org/doi/abs/10.1021/acs.biochem.8b00581
- Min JH, Sarlus H, Harris RA. MAD—microbial (origin of) Alzheimer’s disease hypothesis: from infection and the antimicrobial response to disruption of key copper-based systems. Front Neurosci. 2024;18:1467333. Available from: https://doi.org/10.3389/fnins.2024.1467333
- Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep. 2021;48:5629–45. Available from: https://doi.org/10.1007/s11033-021-06512-9
- Boxer AL, Sperling R. Accelerating Alzheimer’s therapeutic development: the past and future of clinical trials. Cell. 2023;186(22):4757–72. Available from: https://www.cell.com/cell/fulltext/S0092-8674(23)01077-2?dgcid=raven_jbs_aip_email
- Liang T, Zhang Y, Wu S, Chen Q, Wang L. The role of NLRP3 inflammasome in Alzheimer’s disease and potential therapeutic targets. Front Pharmacol. 2022;13:845185. Available from: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.845185/full
- Li YB, Fu Q, Guo M, Du Y, Chen Y, Cheng Y. MicroRNAs: pioneering regulators in Alzheimer’s disease pathogenesis, diagnosis, and therapy. Transl Psychiatry. 2024;14(1):367. Available from: https://doi.org/10.1038/s41398-024-03075-8
- Sery O, Povová J, Zeman T. Molecular mechanisms of Alzheimer’s disease: A review of potential therapeutic targets. Folia Neuropathol. 2013;51(1):1–9. Available from: https://doi.org/10.5114/fn.2013.34190
- Metah MJ, Fitzpatrick AL, Schneider JA. Ancient dementias and overlapping neuropathologies: Therapeutic potential for mixed pathologies. Clin Geriatr Med. 2022;38(4):741–53.
- Vogt NM, Kerby RL, Dill-McFarland KA, Johnson SC. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2021;11(1):10238.
- Nara PL, Sindelar D, Penn MS, Potempa J, Griffin W, Sue T. Porphyromonas gingivalis outer membrane vesicles as the major driver of and explanation for neuropathogenesis, the chlorine hypothesis, iron dyshomeostasis, and salivary lactoferrin in Alzheimer’s disease. J Alzheimers Dis. 2021;82:1417–50. Available from: https://doi.org/10.3233/JAD-210448
- Xie J, Cools L, Van Imschoot G, Van Wonterghem E, Pauwels MJ, Vlaeminck I, Vandenbroucke RE. Helicobacter pylori‐derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3‐C3aR signalling. J Extracell Vesicles. 2023;12(2):12306. Available from: https://doi.org/10.1002/jev2.12306
- Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Haditsch U, Raha D, Griffin C, Holsinger LJ, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5:eaau3333. Available from: https://www.science.org/doi/full/10.1126/sciadv.aau3333
- Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 2013;6:665–77. Available from: https://doi.org/10.3233/JAD-121918
- Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 2002;17:113–8. Available from: https://doi.org/10.1046/j.0902-0055.2001.00100.x
- Leira Y, Dominguez C, Seoane J, Seoane-Romero JM, Pías-Peleteiro JM, Takkouche B, et al. Is periodontal disease associated with Alzheimer's disease? A systematic review with meta-analysis. Neuroepidemiology. 2017;48(1-2):21–31. Available from: https://doi.org/10.1159/000458411
- Nadim R, Tang J, Dilmohamed A, Yuan S, Wu C, Bakre AT, et al. Influence of periodontal disease on risk of dementia: a systematic literature review and a meta-analysis. Eur J Epidemiol. 2020;35:821–33. Available from: https://link.springer.com/article/10.1007/s10654-020-00648-x
- Harding A, Singhrao SK. Periodontitis and dementia: a bidirectional relationship? J Dent Res. 2022;101(3):245–6. Available from: https://doi.org/10.1177/00220345211043461
- Bougea A, Gourzis P. Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine. J Clin Med. 2024;13(16):4661. Available from: https://doi.org/10.3390/jcm13164661

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
