Archives of Depression and Anxiety
A Pilot Study of Transcranial Magnetic Stimulation Effects on Cognitive Distortions and Metacognition in Treatment-resistant Major Depression
Michael J Minzenberg1,2*
1Department of Psychiatry, University of California San Diego School of Medicine, San Diego, CA, USA
2Research Division, Mindful Health Solutions, Oakland, CA, USA
Cite this as
Minzenberg MJ. A Pilot Study of Transcranial Magnetic Stimulation Effects on Cognitive Distortions and Metacognition in Treatment-resistant Major Depression. Arch Depress Anxiety. 2025;11(1):001-004. Available from: 10.17352/2455-5460.000098Copyright
© 2025 Minzenberg MJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Transcranial Magnetic Stimulation (TMS) has well-established effects on the hallmark symptoms of depression (e.g., DSM symptom criteria), yet it remains unknown whether other clinical phenomena are responsive, such as cognitive distortions and metacognitive disturbances that are central to psychological models of depression.
Methods: The author assessed cognitive distortions and metacognition with two validated self-report measures (Cognitive Distortions Questionnaire, CDQ, and Metacognition Questionnaire, MCQ-30, respectively) in the first and final weeks of naturalistic TMS treatment in a continuous series of 20 outpatients with treatment-resistant major depression, along with two weekly self-report symptom measures (PHQ9 and IDS-30-SR).
Results: Analysis of covariance (with Age, Gender and pre-treatment PHQ9 scores as covariates) showed significant effects of Time (p < 0.001), Scale (p < 0.001) and the Time-by-Scale interaction (p = 0.027). In post-hoc one-tailed paired t tests, each scale showed a significant decrease in mean score from First week to Final week. Clinical Responders (n = 10; defined as PHQ9 decrease ≥ 50%), compared to others (n = 10), showed numerically greater mean reductions in CDQ (36% vs. 18%; Cohen’s d = 0.62) and MCQ-30 (8% vs. 2%; d = 0.65). CDQ reductions were moderately correlated with PHQ9 reductions (r = 0.44) and IDS-30-SR reductions (r = 0.45); MCQ-30 reductions were minimally correlated with PHQ9 reductions (r = 0.09) and IDS-30-SR reductions (r = 0.22). Lower baseline CDQ scores were predictive of stronger outcomes on the PHQ9 (r = .62).
Conclusion: TMS may remediate higher-order psychological processes such as cognitive distortions and metacognition in TRD, on a relatively short timescale and somewhat independent of symptom relief. The CDQ in particular may have unique, complementary and sensitive utility in monitoring TMS treatment effects in TRD.
Introduction
Transcranial Magnetic Stimulation (TMS) has well-established efficacy for treatment-resistant major depressive disorder (TRD) and shows considerable promise for a range of other central nervous system disorders [1-4]. The hallmark symptoms of major depression (e.g. those that form the DSM criteria set) are consistently responsive to varied TMS stimulation protocols and brain targets, as measured by diverse structured, validated symptom scales [2,5-7]. However, it remains unknown whether TMS can also modify the varied clinical phenomena that represent central theoretical constructs in influential psychological models of depression [8,9]. Anecdotal clinical experience suggests that many patients report changes in these complex aspects of psychology during the course of TMS treatment, often in a timeframe much shorter than for similar responses to either antidepressant medications or most readily available psychotherapies. More generally, while both biomedical and psychotherapeutic interventions have demonstrated efficacy in treatment-resistant depression (TRD), both families of treatments have emphasized measurement of depression symptoms rather than higher-order meta-cognitive phenomena in this particular clinical population [10]. With these conceptual and empirical issues in mind, the current report describes a pilot study of TMS effects on both the characteristic cognitive distortions that inform models and strategies in Cognitive Behavioral Therapy (CBT) [8] and the metacognitive disturbances that are found in a range of psychiatric disorders [11] and serve as targets for CBT [12] and the basis for metacognitive psychotherapy [9,13].
Methods
Subjects
Twenty outpatients were studied during naturalistic treatment in a private practice setting. This sample size was chosen as it provided more than sufficient power to detect a significant change in a previously reported response of subgroups to a short course of CBT treatment [12]. All provided informed consent for treatment and outcome measures, the study received IRB exemption, and the investigator completed recurring certifications in research ethics and maintained compliance with ethical standards of clinical research. Each patient had a DSM5 diagnosis of major depressive disorder, recurrent, severe without psychotic features (established by the Mini-International Neuropsychiatric Interview with screen (MINI) [14], administered by the author within the month before treatment initiation), and lacked standard clinical contraindications for TMS treatment. Each patient had failed to respond to (and/or tolerate) ≥ 3 adequate antidepressant trials in the current depressive episode. The demographic characteristics included the following: 65% female, 26% male, 9% non-binary; mean age 49.1 ± 16.2 years (range 20 to 75 years); race/ethnicity: 87% White, and 4% each Black, Hispanic, and Asian. All were clinically stable (e.g. no inpatient hospitalizations for at least 12 months at study, nor during the course of TMS treatment and no elevated acute suicide risk). Seventeen patients (87%) were taking antidepressant medications at study, none of which were altered for at least 2 months prior to nor during TMS treatment. 12 patients (60%) were in active, weekly psychotherapy, with diverse psychotherapy modalities. None were in brief, time-limited or Metacognition-directed psychotherapy.
TMS treatment. All were subject to a standard course of TMS treatment, i.e. 35-37 sessions, each session delivering ≥ 3,000 pulses at 10 Hz in 40-pulse trains to left DLPFC target (targeting F3, determined using the Beam F3 method) at 120% of resting motor threshold, determined in the standard manner with single pulses directed to the scalp over M1 to elicit overt movement in the abductor pollicis brevis muscle of the right hand. A Magventure MagPro R30 with Cool-B65 coil was used for stimulation. All subjects were treated with a naturalistic course of TMS, with mid-course adjustments as needed to optimize clinical outcomes. The mean total 10 Hz pulse count for the full treatment course was 119,606 ± 15,203 pulses (range 99,360 to 145,280). Ten patients also had stimulation at 1 Hz to right DLPFC (1800 pulses per session at 120% of resting motor threshold) initiated between session 10-15, with total pulse counts for the full 1 Hz treatment course among the 10 patients with mean 28,600 ± 17,222 pulses (range 5,000 to 68,000). Seven patients also received intermittent theta burst stimulation (600 pulses per session to left DLPFC at 90% of resting motor threshold), with total pulse counts for the full iTBS treatment course among the 7 patients with mean 12,231 ± 5,334 pulses (range 6,600 to 19,800). All stimulation protocols were well-tolerated, with no persisting side effects or reasons for discontinuation, except in one iTBS case in one patient associated with worsening of chronic non-migraine headache, resulting in discontinuation of iTBS after several sessions, while 10 Hz stimulation was continued.
Clinical measures
Cognitive Distortions Questionnaire (CDQ) [15,16].
The 15 items of the CDQ assesses the frequency and intensity of a specific cognitive distortion, e.g. dichotomous thinking, emotional reasoning, magnification/minimization, overgeneralization, and other characteristic targets of cognitive behavioral therapy. The measurement properties of the initial Brazilian Portuguese version in a sample of Brazilian undergraduate students show good internal consistency (Cronbach’s α = .85), convergent validity with self-report measures of depression and anxiety (r = .65, and r = .52, respectively), and discriminate between those endorsing high depression and anxiety and those lacking these symptoms [15,16]. Principal components analysis revealed a single component factor structure [16]. Similar results have been reported in an Anglophone Australian sample [17]. The CDQ was administered twice, within the first two and final two sessions of treatment (mean interval 80 ± 28.5 days for both CDQ and MCQ-30).
Metacognition Questionnaire - 30 item (MCQ-30) [18].
The 30-item MCQ is a short version of the original 65-item scale, and assesses varied metacognitive phenomena related to mood and anxiety syndromes, e.g. “Worrying helps me to avoid problems in the future,” “I should be in control of my thoughts all of the time,” “I constantly examine my thoughts.” Items are scored on a 4-point Likert scale from “Do not agree” to “Agree very much.” It has good internal consistency, convergent validity with obsessive and compulsive symptoms, pathological worry and trait anxiety, and good test-retest reliability [18]. A large meta-analytic study has found widespread disturbances in metacognition using the MCQ-30 across diverse psychiatric diagnoses, including major depressive disorder [11]. The MCQ-30 is sensitive to CBT treatment for depression [12] and Generalized Anxiety Disorder [19] and psychotherapy explicitly targeting metacognition has efficacy in depression [13]. The MCQ-30 was administered twice, within the first two and final two sessions of treatment, on the same days as the CDQ.
Personal Health Questionnaire – 9 item version (PHQ9) [20].
The Patient Health Questionnaire (PHQ) 9-item scale is the self-administered depression module from the PRIME-MD diagnostic instrument for common mental disorders. Each of the nine DSM-IV criteria is scored from “0” (not at all) to “3” (nearly every day). The PHQ9 shows good performance in detection of major depressive disorder and compares favorably with major depressive disorder diagnosis derived from validated, structured diagnostic interviews [21]. It is also sensitive to TMS treatment effects [7]. The PHQ9 was administered prior to (within one week of) TMS treatment initiation and then weekly through the final week of treatment.
Inventory of Depressive Symptoms 30 Item Self-Report (IDS-30-SR).
The IDS-30-SR is a 30 item self-report measure of depressive symptom severity [22,23]. It includes all 9 DSM-IV and DSM5 criterion symptoms, as well as commonly associated symptoms (e.g. anxiety, irritability) and items relevant to melancholic, or atypical symptom features. The IDS-30-SR is sensitive to TMS treatment [1]. The IDS-30-SR was administered prior to (within one week of) TMS treatment initiation and then weekly through the final week of treatment.
Results
Analysis of covariance (ANCOVA) was conducted with Time (First Week, Final Week) and Scale (CDQ, MCQ, PHQ9, IDS-30-SR) entered as within-subject variables, and Age, Gender and pre-treatment PHQ9 scores as covariates. This analysis revealed a statistically significant effects of Time F (1,16) = 28.0, p < 0.001, Scale F(3,14) = 287.9, p<0.001, and the Time-by-Scale interaction F(3,14) = 3.93, p = 0.027.
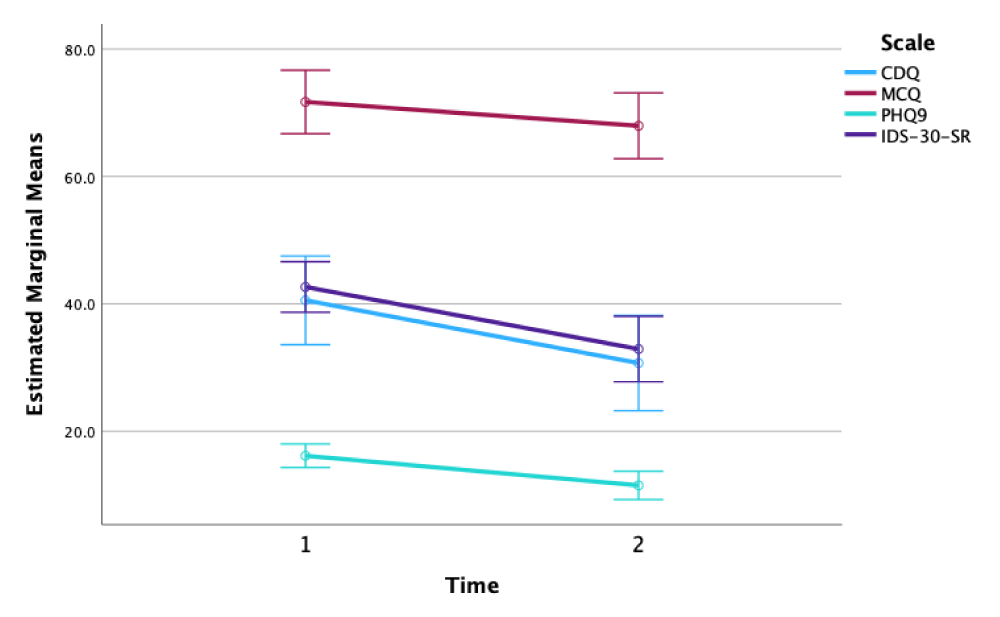
All 4 clinical scales showed decreases from first scores to final scores, which were each statistically significant in one-tailed paired T tests after correction using the False Discovery Rate (FDR) method (p < 0.0327; see also Figure 1 and Table 1). Mean total scale scores decreased from first week to final week on the CDQ (24%, Cohen’s d = 1.09; t = 4.89, df = 19, p<0.0005), MCQ-30 (5%, d = 0.53; t = 2.37, df = 19, p = 0.014), PHQ9 (29%, d = 0.83; t = 3.72, df = 19, p = 0.0005) and IDS-30-SR (23%, d = 0.85; t = 3.40, df = 19, p = 0.0013).
Clinical Responders (n = 10; defined as a PHQ-9 decrease ≥ 50%), compared to Others (n = 10), demonstrated greater mean reductions in CDQ scores (36% vs. 18%; d = 0.62; t = -1.39, df = 18, p = 0.090) and MCQ-30 (8% vs. 2%; d = 0.65; t = -1.41, df = 15, p = 0.090); These subgroup differences were not statistically significant, likely due to prohibitively small sample sizes in each subgroup. CDQ reductions were moderately correlated with reductions in PHQ9 (Pearson’s r = 0.44) and IDS-30-SR (r = 0.45); in contrast, MCQ-30 reductions were minimally correlated with reductions in PHQ9 (r = 0.09) and IDS-30-SR (r = 0.22). Finally, baseline (i.e., first-week) CDQ scores were strongly inversely correlated with decreases in PHQ9 scores at treatment end (r = –0.615), i.e. lower baseline CDQ scores predicted greater treatment benefits measured by PHQ9.
Discussion
As noted above, empirical evaluation of clinical constructs that are fundamental to cognitive models of depression has been largely neglected in studies of biomedical treatments such as TMS, and similarly in the study of any intervention modality in treatment-resistant clinical populations. The present pilot study evaluated changes in cognitive distortions and metacognitive phenomena (with validated, quantitative self-report scales) during a course of TMS treatment in patients with TRD, finding significant improvements in these psychological phenomena with an average treatment interval of less than 3 months of treatment. The measure of cognitive distortions in particular showed a magnitude of improvement that was quite similar in magnitude to (and correlated with) contemporaneous changes in 2 standard depression symptom scales, and was stronger in those who met standard criteria for clinical responder status in TMS. While this study was not a randomized controlled clinical trial, and therefore the possible influence of patient expectancies cannot be ruled out, these two findings both suggest that the apparent benefits for cognitive distortions was unrelated to factors other than the intervention itself. The observation that neither the CDQ nor MCQ changes were strongly correlated with changes in the 2 standard depression symptom scales nevertheless suggests that improvements in cognitive distortions and metacognition may occur somewhat independently of DSM symptom change. Taken together, these novel findings suggest that even in patients with TRD, TMS may improve core higher-order cognitive phenomena that have been an integral part of cognitive models of depression, and within a timeframe comparable to other biomedical treatment approaches and with CBT itself. It remains unknown how the magnitude of these improvements compare between these diverse types of treatments for depression.
Study limitations include the lack of blinded clinical assessments, or clinician-administered severity ratings. In addition, the sample is relatively small and potentially underpowered, e.g. to detect differences between subgroups of patients. Finally, the potential influence of ongoing antidepressant therapy and external variables (e.g., social support) were not assessed here.
Further study should aim for larger and more diverse patient samples, and randomized controlled, double-blind trials; monitor these changes with higher temporal resolution (i.e. more frequent sampling) to ascertain the temporal development and durability of these effects during treatment; disambiguate the direct effects of TMS, and which of the varied TMS protocols exert the strongest effects; compare TMS effects directly with other treatment modalities (e.g., antidepressant medications, CBT), and determine whether pre-treatment clinical factors may serve as effective predictors of treatment response. These advances may help optimize treatment strategies for a clinically significant population with global health implications.
- Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–92. Available from: https://doi.org/10.1016/s0140-6736(18)30295-2
- George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13–8. Available from: https://doi.org/10.1097/yco.0b013e32835ab46d
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–206. Available from: https://doi.org/10.1016/j.clinph.2014.05.021
- Minzenberg MJ, Yoon JH. Transcranial Magnetic Stimulation: A Clinical Primer for Nonexperts. J Psychiatr Pract. 2020;26(5):423–8. Available from: https://doi.org/10.1097/pra.0000000000000490
- Brini S, Brudasca NI, Hodkinson A, Kaluzinska K, Wach A, Storman D, et al. Efficacy and safety of transcranial magnetic stimulation for treating major depressive disorder: An umbrella review and re-analysis of published meta-analyses of randomised controlled trials. Clin Psychol Rev. 2021;100:102236. Available from: https://doi.org/10.1016/j.cpr.2022.102236
- Leuchter MK, Citrenbaum C, Wilson AC, Tibbe TD, Jackson NJ, Krantz DE, et al. A comparison of self- and observer-rated scales for detecting clinical improvement during repetitive transcranial stimulation (rTMS) treatment of depression. Psychiatry Res. 2023;330:115608. Available from: https://doi.org/10.1017/s1041610224000462
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016;9(3):336–46. Available from: https://doi.org/10.1016/j.brs.2016.03.010
- Beck AT, Haigh EA. Advances in cognitive theory and therapy: the generic cognitive model. Annu Rev Clin Psychol. 2014;10:1-24. Available from: https://doi.org/10.1146/annurev-clinpsy-032813-153734
- Wells A. Emotional disorders and metacognition: Innovative cognitive therapy. New York: Wiley; 2000.
- van Bronswijk S, Moopen N, Beijers L, Ruhe HG, Peeters F. Effectiveness of psychotherapy for treatment-resistant depression: a meta-analysis and meta-regression. Psychol Med. 2019;49(3):366–79. Available from: https://doi.org/10.1017/s003329171800199x
- Sun X, Zhu C, So SHW. Dysfunctional metacognition across psychopathologies: A meta-analytic review. Eur Psychiatry. 2017;45:139–53. Available from: https://doi.org/10.1016/j.eurpsy.2017.05.029
- Gupta A, Kumari S. Effect of CBT on Metacognitive Beliefs in Depressive Disorders. Turk J Psychiatry. 2023;34(2):80–8. Available from: https://doi.org/10.5080/u26398
- Normann N, van Emmerik AA, Morina N. The efficacy of metacognitive therapy for anxiety and depression: A meta-analytic review. Depress Anxiety. 2014;31(5):402–11. Available from: https://doi.org/10.1002/da.22273
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. Available from: https://pubmed.ncbi.nlm.nih.gov/9881538/
- de Oliveira IR. Introducing the Cognitive Distortions Questionnaire. In: de Oliveira IR, editor. Trial-based cognitive therapy: A manual for clinicians. New York: Routledge; 2015;25–40.
- de Oliveira IR, Seixas C, Osorio FL, Crippa JAS, de Abreu JN, Menezes IG, et al. Evaluation of the psychometric properties of the Cognitive Distortions Questionnaire (CD-Quest) in a sample of undergraduate students. Innov Clin Neurosci. 2015;12:20–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26351620/
- Kostoglou S, Pidgeon AM. The Cognitive Distortions Questionnaire: Psychometric validation for an Australian population. Aust J Psychol. 2016;68(2):123–9. Available from: https://doi.org/10.1111/ajpy.12101
- Wells A, Cartwright-Hatton S. A short form of the metacognitions questionnaire: Properties of the MCQ-30. Behav Res Ther. 2004;42(4):385–96. Available from: https://doi.org/10.1016/s0005-7967(03)00147-5
- Strand ER, Hjemdal O, Anyan F, Nordahl H, Nordahl HM. Change in interpersonal problems and metacognitive beliefs as predictors of improvement in patients with generalized anxiety disorder. Clin Psychol Psychother. 2023;30(4):842–51. Available from: https://doi.org/10.1002/cpp.2841
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. Available from: https://doi.org/10.1046/j.1525-1497.2001.016009606.x
- Levis B, Benedetti A, Thombs BD; DEPRESsion Screening Data (DEPRESSD) Collaboration. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. BMJ. 2019;365:l1476. Available from: https://doi.org/10.1136/bmj.l1476
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Res. 1986;18(1):65–87. Available from: https://doi.org/10.1016/0165-1781(86)90060-0
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26(3):477–86. Available from: https://doi.org/10.1017/s0033291700035558
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
