Archives of Depression and Anxiety
“Perrotta Border-bipolar Profile Diagnostic Questionnaire” (PBBD-Q): Development, Regulation, and Validation of a Psychometric Instrument for the Unified Diagnosis of the Psychopathological Condition in Adults
Department of Human and Social Sciences, University of Merchants, Piazza Mattei 10, 00186 Rome, Italy
Author and article information
Cite this as
Perrotta G, Eleuteri S. “Perrotta Border-bipolar Profile Diagnostic Questionnaire” (PBBD-Q): Development, Regulation, and Validation of a Psychometric Instrument for the Unified Diagnosis of the Psychopathological Condition in Adults. Arch Depress Anxiety. 2025; 11(1): 032-038. Available from: 10.17352/2455-5460.000103
Copyright License
© 2025 Perrotta G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Abstract
Introduction: In the literature, the high risk of diagnostic error in diagnoses of borderline disorder and bipolar disorder, due to overlapping part of symptoms, is well known. There is a need to validate a psychometric instrument capable of reducing this risk.
Materials and methods: A theory, model, scale and questionnaire related to the unified diagnosis of Border-Bipolar psychopathological condition (Perrotta Border-Bipolar Profile Diagnostic Questionnaire, PBBD-Q) was generated to be administered to a selected population; however, since there is no psychometric instrument capable of performing this analysis, the data were compared with the outcomes of the PICI-3-TA columns related to the disorders under investigation, to validate the proposed psychometric instrument.
Results: In this study, a population of 232 individuals (96 males and 136 females), aged between 18 and 68 years (M: 39.4; SD: 3.1), was selected. KMO and EFA all show values above 0.500, which is still considered adequate. Statistical comparison between PBBD-Q and PICI-3-TA showed good significance (p = 0.017 and W = 0.878), with a fair correlation matrix (r = 0.866). Statistical analysis showed that the psychometric test has a well-defined and stable construct, with the variables well represented and positively correlated with another construct already validated.
Conclusion: PBBD-Q is a valid, efficient, and effective psychometric tool to identify the exact unitary diagnosis of the Border-Bipolar psychopathological condition.
Abbreviations
PBBD-Q: Perrotta Border-Bipolar Profile Diagnostic Questionnaire; BPD: Borderline personality disorder; BD: Bipolar Disorder; PICI-3: Perrotta Integrative Clinical Interviews – 3; DSM-5-TR: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision
Background
Introduction
In psychopathology, one of the most complex challenges is offered by the diagnostic parallelism between patients with borderline personality disorder (BPD) and those with bipolar disorder (BD), as many of the symptoms seem to overlap, generating confusion. Such assessment, however, is often subject to subjective interpretive judgment based on clinical history, narrative during interviews, and psychodiagnostic outcomes, which to date have never really defined the diagnostic boundary, although the two nosographic categories are distinct [1-7].
The DSM-5-TR defines BPD as one of the 4 cluster B personality disorders characterized by a consistent, pervasive, and enduring pattern of instability in interpersonal relationships, self-image, and affectivity, and marked impulsivity [7-12].
The DSM-5-TR defines BD as a spectrum of mood disorders that originated from the generic term "manic-depressive psychosis" and consisted of syndromes of psychiatric interest characterized by an alternation between the two counter-polar conditions of psychic activity, its excitement (mania) and on the reverse its inhibition (depression), combined with a wide range of neurotic symptoms and psychotic alterations in thinking [7,13-23].
Perrotta Integrative Clinical Interviews-3 (PICI-3) defines borderline personality disorder (BPD, category No. 11) as a habitual, stable, persistent, and pervasive pattern, with onset around age 8 (but evolving structurally into adolescence and adulthood), characterized by emotional instability, sudden mood swings, and impulsivity. The PICI-3 defines bipolarism (BpD, category No. 7) as a habitual, stable, persistent, and pervasive pattern, with onset between the ages of 5 and 10 years (but evolves structurally into adolescence), characterized by abrupt mood fluctuations, manic and/or depressive states, and/or abrupt alternation and emotional instability [7,24].
According to the DSM-5-TR nosographic formulation, these 2 disorders can coexist in the same patient, as a mood disorder (bipolarism) is grafted into the personality disorder (border). It happens that a borderline subject presents alongside the affective instability proper to the disorder, true depressive, or manic episodes. In such a case, we have comorbidity between borderline disorder and bipolar disorder. For PICI-3, however, the topic is quite complex, as it intersects the combination of manic, bipolar, and borderline traits in its answer, as it can be inferred from the above structure that most of the commonalities between BPD and BPD are predominantly with manic tendency [7, 25-29].
To meet the clinical needs of nosographic organization, and to reduce the risk of diagnostic errors, the Perrotta Border-Bipolar Diagnostic Questionnaire (PBBD-Q) was developed (based on the PICI-3) [30]. In this study, analyses are conducted to confirm the validation of the psychometric instrument.
Aim
A validation study was conducted to determine whether the proposed psychometric instrument (PBBD-Q) is capable of being reliable, efficient, effective, and valid for the unified diagnosis of the Border-Bipolar psychopathological condition. Therefore, the present discussion aims to try to determine whether, in the current state of scientific knowledge, it is possible to validate the proposed psychometric instrument concerning the specific topic, according to the author's understanding of the present study's model.
Materials and methods
Study design, consent, and data protection procedures
Development, adjustment, and validation of a psychometric instrument capable of performing the unified diagnosis of the Border-Bipolar psychopathological condition (PBBD-Q), through population sample administration to test its effectiveness, efficiency, and validity. Subjects who gave regular informed consent agreements were recruited; moreover, these subjects requested and obtained from Giulio Perrotta, as the sole examiner and project manager, not to meet the other study collaborators, thus remaining completely anonymous. The subjects who participated in the study requested and obtained that Giulio Perrotta be the sole examiner during the therapeutic sessions and that all other authors be aware of the participants' data in an exclusively anonymous form.
Materials and methods
PBBD-Q represents, in international literature, the first modern questionnaire capable of framing a unified diagnosis concerning the shared and specific symptoms of borderline and bipolar disorder diagnoses, identifying 6 different types in the first case and 5 different types in the second case. The method used consists of two consecutive operations: the first is related to the clinical interview, based on narrative anamnestic and documentary evidence, with an interview regarding the emotional and perceptual-reactive experience of the patient, according to the PHE-Model updated to the new version PHEM-2 [31]; the second is related to the administration of the PBBD-Q in comparison with the 5 scales related to the same disorders (6, 7, 8, 10, and 11) in the PICI-3-TA, to enable a comprehensive statistical analysis for the validation of the PBBD-Q. The following statistical analyses were performed: descriptive profile, comparison of means, KMO (measure of sampling adequacy - MSA), χ² (Barlett's test of sphericity), EFA (exploratory factor analysis, using the "maximum likelihood" extraction method in combination with a "promax" rotation), Pearson's r (BORI-BAT correlation matrix), W (Shapiro-Wilk normality test), paired T-test (with 95% confidence interval) and multivariate regression model. IBM SPSS software (28th edition) was used. p < 0.05 was considered statistically significant. The results are consistent and in accordance with the rules of the Standards for Educational & Psychological Testing (2014 Edition). The stages of the research were divided as follows: 1. Selection of the population sample, according to the parameters given in the next paragraph. 2. Clinical interview with each population group, as indicated in the next paragraph. 3. Administration of psychometric tests. 4. Data processing after administration. 5. Comparison of the data obtained.
Setting and participants
Inclusive criteria for the selection of the population are: 1) Age between 18 years and 68 years; 2) Italian nationality; 3) Bipolar diagnosis, borderline diagnosis, or mixed diagnosis confirmed with a medical certificate issued by a public or private contracted health facility; 4) Absence of neurodegenerative disorders or severe genetic diseases capable of impairing cognitive functioning. Exclusive criteria for the selection of the population are: 1) Age < 18 years and > 68 years; 2) foreign nationality; 3) Absence of bipolar diagnosis, borderline diagnosis, or mixed diagnosis confirmed by a medical certificate issued by a contracted public or private health facility or confirmation of diagnosis but by a simple, non-contracted private health facility; 4) Presence of neurodegenerative disorders or severe genetic diseases capable of impairing cognitive functioning. The chosen setting, tender standing during the protracted pandemic period (already in progress since the beginning of the present research), is the online platform via Skype and WhatsApp Video Calls, both for clinical interviews and administration. The questionnaire was administered directly by Giulio Perrotta, via the previous online platforms, during dedicated meetings, using Google Forms, with a link sent at least two hours before the meeting. The language used for data collection and the questionnaires is exclusively Italian. The questionnaire was then translated into English for publication purposes. The present research work was carried out from June 2021 to December 2023. All participants were guaranteed anonymity, and the ethical requirements of the Declaration of Helsinki were met. Because the research is not funded by anyone, it is free of conflicts of interest. The sample of the selected population is 232 participants (96/m; 136/f) for the entire study (M: 39.4; SD: 3.1). The drop-out rate was 0/232 (0.0%) (Table 1).
Results
Development and regulation of the questionnaire (PBBD-Q)
PBBD-Q was developed, structured into 36 items with dichotomous yes/no (Y/N) responses, with 9 progressive items for 5 categories (items 1-9 for manic traits, items 10-18 for bipolar traits, items 19-27 for depressive traits, items 28-36 for emotive traits, and items 37-45 for borderline traits) and 4 columns (A, B, C, D) corresponding to the 4 time reference periods (1-2-3-4 months) from the day of administration. The therapist will manage the administration and the patient should answer the questions, with his/her support, choosing from 2 possible answers (Y for affirmative answer and N for negative answer) and referring to his/her personal experience of the last month of life (column A), of the month of life preceding that referred to column A (column B), of the month of life still preceding that referred to column B (column C), and finally of the month of life preceding that referred to column C (column D). An affirmative answer will be initialed when the behavior described in the item has a frequency of at least 7 out of 30 days. It is necessary, therefore, for each item to be answered 4 times to cover the last 4 months of life. Missing responses are not allowed [30].
Court study
The cohort study of the selected population sample shows that the female component accounts for nearly 60.0% of the total sample, with a greater preponderance in the 38-47 age group (35.3%) and 18-27 years (24.3%), to decrease progressively with advancing age; on the other hand, the shares are represented in increasing majorities from borderline disorder (25.9%) to bipolar disorder (31.0%), with the greatest prevalence in mixed disorder (43.1%).
Validation of the questionnaire (PBBD-Q)
Comparison of test structures:
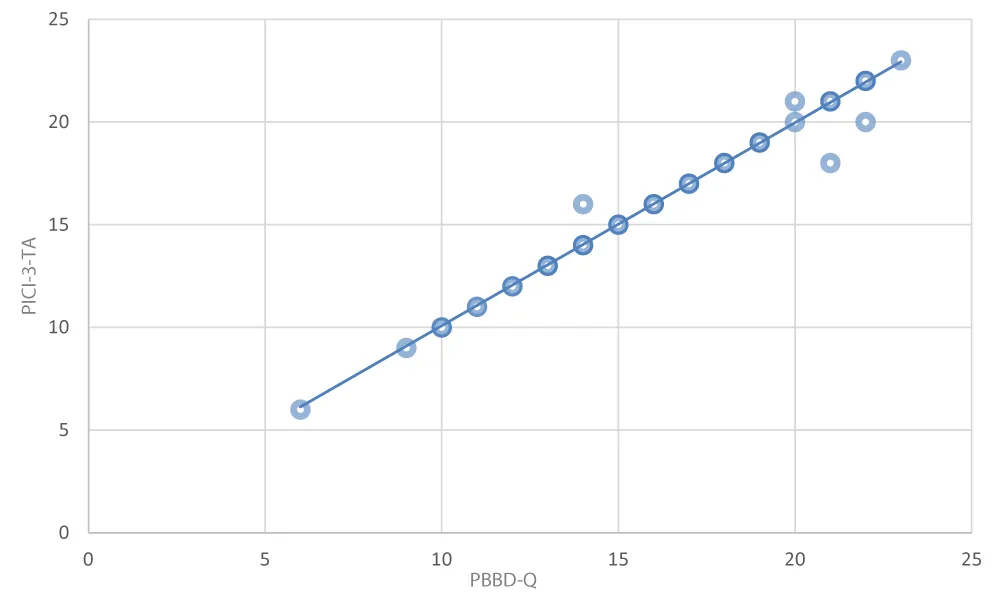
Introduction: Structurally, there is no questionnaire in the literature capable of investigating the relationship between borderline and bipolar disorder, and therefore the last way to validate the PBBD-Q is to compare the outcomes with those of the already validated PICI-3-TA [24], concerning the manic (No. 6), bipolar (No. 7), emotive (No. 8), depressive (No. 10) and borderline (No. 11) scales, both having the same structure (on a 0-9 basis) and functioning (identification of the dysfunctional traits of the specific disorder). Below is the comparison of the items of the two questionnaires compared (PBBD-Q / PICI-3-TA) (Table 2).
The comparison was then made, for each patient, by summing the individual values of the comparison items with scale values 0-1 (0 for no and 1 for yes), for a maximum total of 9/9 per individual scale (manic, bipolar, depressive, and borderline).
The results are compared in the following graph (Figures 1,2):
Measure of sampling adequacy, Barlett's test of sphericity and exploratory factor analysis: Table 3 shows the data for the statistical analyses carried out about KMO (Measure of Sampling Adequacy - MSA), χ² (Barlett's Test of Sphericity), EFA (Exploratory Factor Analysis), as indicated in the Methods section, for the PBBD-Q items and for the totals of its individual sections. The content validity was verified by a group of 40 experts, including psychologists, psychotherapists, and psychiatrists, 20 men and 20 women, obtaining an average Cronbach's alpha greater than 0.800.
Discussion
PBBD-Q is a psychometric instrument designed to address the need to ensure better diagnostic framing in those patients who present with both borderline and bipolar symptoms, decreasing the risk of diagnostic error and identifying in detail the specific type of disorder, in its subtypes. This diagnostic revolution is in the groove of the innovative PICI-3 model, which analyzes both functional and dysfunctional traits, emphasizing not the diagnosis of status (rigid) but the diagnosis of personological characteristics (elastic), changeable over time by its very nature. Even in this model, however, the need for reorganization of the border-bipolar diagnosis had failed to be fully met, leaving (subjective) space for the therapist from time to time, even in the presence of scale over-elevations, often failing to consider that some features were common to the various disorders and thus risking false over-elevation. PBBD-Q corrects this interpretive bias by redefining the application domains, respecting the basic diagnosis and correcting for individual characteristics, and adding the identification of dysfunctional subtypes, both in the hypothesis of borderline disorder and bipolar disorder. The diagnoses obtained with the PICI-3-TA were totally confirmed by the PBBD-Q, but the interpretive frameworks were reshaped to avoid the risk of suspicious or falsified over-elevations, or otherwise capable of diverting the therapist's attention to the real problem at the personological matrix. Statistical analysis confirmed what was hoped for, namely, that the PBBD-Q has a well-defined and stable construct, the variables are well represented, and it is positively correlated with another construct that has already been validated. The new diagnostic framing, therefore, does not distort the PICI-3-TA formulation but rather adds greater precision both structurally (identifying the exact nosographic diagnosis without risk of overlap or excessive reductionism) and functionally (identifying dysfunctional subtypes).
Limitations, implications for clinical practice, and prospects
In this validation analysis, the only limitation found relates to test comparison, as there is no validated psychometric instrument in the literature that meets the need for border-bipolar framing. Using the PICI-3 was a necessity determined by this limitation, despite the fact, however, that the latter is a psychometric instrument, in its third version, validated, and therefore efficient and effective, both concerning subscales and overall score. However, during validation, corrections were made to some items in the PICI to center the object of investigation and the topic of interest, but without distorting its structure and operation. These technical adjustments will then be the subject of a revision of the PICI-3 to improve its internal validity. The clinical implication from this validation is undoubtedly crucial for the diagnostic future of these patients and of their treatment, both in terms of psychotherapy and psychopharmacology. Prospects are geared toward a study with a larger population sample, also considering the results at follow-up, at 6, 12, 18, and 24 months, because of the neurobiological findings.
Conclusion
PBBD-Q is a valid, efficient and effective psychometric tool to identify the exact unitary diagnosis of the Border-Bipolar psychopathological condition, being capable of not distorting the already validated PICI-3-TA formulation and adding greater precision to the final diagnosis, both in structural terms (identifying the exact nosographic diagnosis without risk of overlapping or excessive reductionism) and in functional terms (identifying dysfunctional subtypes), especially from a diagnostic and therapeutic perspective.
Ethics approval and consent to partecipate
This study was waived for ethical review and approval because all participants were assured compliance with the ethical requirements of the Charter of Human Rights, the Declaration of Helsinki in its most recent version, the Oviedo Convention, the guidelines of the National Bioethics Committee, the standards of "Good Clinical Practice" (GCP) in the most recent version, the relevant national and international ethical codes, as well as the fundamental principles of state law and international laws according to the updated guidelines on observational studies and clinical trial studies. Pursuant to Legislative Decree No. 52/2019 and Law No. 3/2018, this research does not require the prior opinion of an ethics committee, in implementation of Regulation (EU) No. 536/2014 and in accordance with Regulation (EU) 2017/745, the Declaration of Helsinki and the Oviedo Convention, since the scientific research contained in the manuscript: (a) does not concern new or already marketed drugs or medical devices; (b) does not involve the administration of a new or already marketed drug or medical device; (c) does not have commercial purposes; (d) is not sponsored or funded; (e) participants have signed the informed consent and data processing, in compliance with applicable national and EU regulations; (f) refers to non-interventional and observational-comparative diagnostic topics; (g) the population sample was collected at a date before the start of this study and is part of a private and non-public database.
Informed consent statement
Subjects who gave regular informed consent agreements were recruited; moreover, these subjects requested and obtained from GP, as the sole examiner and project manager, not to meet the other study collaborators, thus remaining completely anonymous.
Data availability statement
The subjects who participated in the study requested and obtained that GP be the sole examiner during the therapeutic sessions and that all other authors be aware of the participants' data in an exclusively anonymous form.
Authors' contributions
The authors who contributed to the work are 2. Giulio Perrotta designed the manuscript, carried out the experiment, and drafted the individual sections as a whole, performing statistical analyses. Stefano Eleuteri drafted the introductory structure of the manuscript. Giulio Perrotta is the creator of the theory, model, scale, and questionnaire, and the sole owner of the intellectual and economic property rights. The authors have read and approved the final manuscript.
References
- Perrotta G. Psicologia clinica. Luxco Ed.; 2019. Available from: https://www.unilibro.it/libro/perrotta-giulio/psicologia-clinica/9782902114023
- Hales RE, Yudofsky SC, Roberts LW. Manuale di Psichiatria. American Psychiatric Publishing. Edra Ed.; 2015. Available from: https://books.google.co.in/books/about/Manuale_di_psichiatria.html?id=wwMhCwAAQBAJ&redir_esc=y
- Kaplan BJ, Sadock VA. Sinossi di Psichiatria. Piccin; 2018. Available from: https://www.piccin.it/it/psichiatria/2069-sinossi-di-psichiatria-9788829927739.html
- Bressi C, Invernizzi G. Psichiatria e Psicologia Clinica. McGraw-Hill Ed.; 2017. Available from: https://www.mheducation.it/manuale-di-psichiatria-e-psicologia-clinica-5-ed-9788838639968-italy
- Mucci C. Corpi borderline. Raffaello Cortina Ed.; 2020. Available from: https://www.raffaellocortina.it/scheda-libro/clara-mucci/corpi-borderline-9788832851427-3110.html
- American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders. 5th ed., text rev. (DSM-5-TR). APA; 2022. Available from: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425787
- Fornaro M, Orsolini L, Marini S, De Berardis D, Perna G, Valchera A, et al. The prevalence and predictors of bipolar and borderline personality disorders comorbidity: Systematic review and meta-analysis. J Affect Disord. 2016;195:105–18. Available from: https://doi.org/10.1016/j.jad.2016.01.040
- Gunderson JG. Borderline personality disorder: a clinical guide. APA. 2001;932(1):61–77. Available from: https://doi.org/10.1111/j.1749-6632.2001.tb05798.x
- Perrotta G. Borderline personality disorder: definition, differential diagnosis, clinical contexts, and therapeutic approaches. Ann Psychiatry Treatm. 2020;4(1):43–56. Available from: https://www.researchgate.net/publication/343908101_Borderline_personality_disorder_Definition_differential_diagnosis_clinical_contexts_and_therapeutic_approaches
- Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. The Lancet. 2011;377(9759):74–84. Available from: https://doi.org/10.1016/s0140-6736(10)61422-5
- Daros AR, Williams GE. A meta-analysis and systematic review of emotion-regulation strategies in borderline personality disorder. Harv Rev Psychiatry. 2019;27(4):217–32. Available from: https://doi.org/10.1097/hrp.0000000000000212
- Gunderson JG. Borderline personality disorder. N Engl J Med. 2011;364(21):2037–42. Available from: https://doi.org/10.1056/nejmcp1007358
- Perrotta G. Bipolar disorder: definition, differential diagnosis, clinical contexts, and therapeutic approaches. J Neurosci Neurol Surg. 2019;5(1):14–9. Available from: https://doi.org/10.31579/2578-8868/097
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–52. Available from: https://doi.org/10.1001/archpsyc.64.5.543
- Diflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437–52. Available from: https://doi.org/10.3109/09540261.2010.514601
- Baldassano CF, Marangell LB, Gyulai L, Ghaemi SN, Joffe H, Kim DR, et al. Gender differences in bipolar disorder: retrospective data from the first 500 STEP-BD participants. Bipolar Disord. 2005;7(5):465–70. Available from: https://doi.org/10.1111/j.1399-5618.2005.00237.x
- De Prisco M, Oliva V, Fico G, Radua J, Grande I, Roberto N, et al. Emotion dysregulation in bipolar disorder compared to other mental illnesses: a systematic review and meta-analysis. Psychol Med. 2023;53(16):7484–503. Available from: https://doi.org/10.1017/s003329172300243x
- Bezerra-Filho S, Galvão-de Almeida A, Studart P, Rocha MV, Lopes FL, Miranda-Scippa Â. Personality disorders in euthymic bipolar patients: a systematic review. Braz J Psychiatry. 2015;37(2):162–7. Available from: https://doi.org/10.1590/1516-4446-2014-1459
- Dominiak M, Jazdzyk P, Antosik-Wojcinska AZ, Konopko M, Bieńkowski P, Świȩcicki Ł, et al. The impact of bipolar spectrum disorders on professional functioning: a systematic review. Front Psychiatry. 2022;13:951008. Available from: https://doi.org/10.3389/fpsyt.2022.951008
- Ferreira AC, de Lima Osorio F. Peripheral oxytocin concentrations in psychiatric disorders – a systematic review and meta-analysis: further evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2022;117:110561. Available from: https://doi.org/10.1016/j.pnpbp.2022.110561
- Plans L, Barrot C, Nieto E, Rios J, Schulze TG, Papiol S, et al. Association between completed suicide and bipolar disorder: a systematic review of the literature. J Affect Disord. 2019;242:111–22. Available from: https://doi.org/10.1016/j.jad.2018.08.054
- Fornaro M, Orsolini L, Marini S, De Berardis D, Perna G, Valchera A, et al. The prevalence and predictors of bipolar and borderline personality disorders comorbidity: systematic review and meta-analysis. J Affect Disord. 2016;195:105–18. Available from: https://doi.org/10.1016/j.jad.2016.01.040
- Lyssenko L, Schmahl C, Bockhacker L, Vonderlin R, Bohus M, Kleindienst N, et al. Dissociation in psychiatric disorders: a meta-analysis of studies using the Dissociative Experiences Scale. Am J Psychiatry. 2018;175(1):37–46. Available from: https://doi.org/10.1176/appi.ajp.2017.17010025
- Perrotta G. Perrotta Integrative Clinical Interviews-3 (PICI-3): development, regulation, updation, and validation of the psychometric instrument for the identification of functional and dysfunctional personality traits and diagnosis of psychopathological disorders, for children (8–10 years), preadolescents (11–13 years), adolescents (14–18 years), adults (19–69 years), and elders (70–90 years). Ibrain. 2024;1–18. Available from: https://doi.org/10.1002/ibra.12148
- Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3–7. Available from: https://doi.org/10.1097/nmd.0000000000000225
- Parker G, Bayes A, Spoelma MJ. Why might bipolar disorder and borderline personality disorder be bonded? J Psychiatr Res. 2022;150:214–8. Available from: https://doi.org/10.1016/j.jpsychires.2022.03.051
- Betzler F, Stover LA, Sterzer P, Köhler S. Mixed states in bipolar disorder – changes in DSM-5 and current treatment recommendations. Int J Psychiatry Clin Pract. 2017;21(4):244–58. Available from: https://doi.org/10.1080/13651501.2017.1311921
- Friborg O, Martinsen EW, Martinussen M, Kaiser S, Overgård KT, Rosenvinge JH. Comorbidity of personality disorders in mood disorders: a meta-analytic review of 122 studies from 1988 to 2010. J Affect Disord. 2014;152–154:1–11. Available from: https://doi.org/10.1016/j.jad.2013.08.023
- Rodriguez AM, Hogg B, Gardoki-Souto I, Valiente-Gómez A, Trabsa A, Mosquera D, et al. Clinical features, neuropsychology and neuroimaging in bipolar and borderline personality disorder: a systematic review of cross-diagnostic studies. Front Psychiatry. 2021;12:681876. Available from: https://doi.org/10.3389/fpsyt.2021.681876
- Perrotta G, Grilli S, Eleuteri S, Petruccelli I. Diagnostic parallels between borderline and bipolar patients in psychopathology: similarities, differences, comorbidities, neural correlates, and a new proposal for the Perrotta Border-Bipolar Profile Diagnostic Questionnaire. Ibrain. 2025;11(3):306–18. Available from: https://doi.org/10.1002/ibra.70001
- Perrotta G, Basiletti V, Eleuteri S. The “Human Emotions” and the new “Perrotta Human Emotions Model” (PHEM-2): structural and functional updates to the first model. Open J Trauma. 2023;7(1):22–34. Available from: https://doi.org/10.17352/ojt.000043
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
