Journal of Addiction Medicine and Therapeutic Science
Antidepressants Drugs and Addiction Treatment: A development view and a technological patent landscape of drugs and compositions
1School of Chemistry, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
2National Institute of Industrial Property, Rio de Janeiro, Brazil
Author and article information
Cite this as
De Oliveira AM, Rodrigues Schumacher SDO, De Souza Antunes AM (2021) Antidepressants Drugs and Addiction Treatment: A development view and a technological patent landscape of drugs and compositions. J Addict Med Ther Sci. 2021; 7(2): 020-025. Available from: 10.17352/2455-3484.000051
Copyright License
© 2021 De Oliveira AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: According to the World Health Organization, about 76-85% of subjects in low- and middle-income countries suffer with a mental disorder. From them the most prevalent are anxiety and depression ones, with an increase in the number of cases during the COVID-19 pandemic. Antidepressants are drugs used to treat depression, but they are also used to treat subjects addicted to drugs. The development of new technologies/drugs/compositions is important for promoting access not only to drugs, but better drugs.
The present study aims: 1. To carry out a survey of drugs under active development used to depression and dependency treatment (at least); 2. To evaluate, through the patent landscape, the patent documents filed in the past 10 years related to this class of drugs (antidepressants) in the context of technological scenario and 3. To promote a discussion about drugs used as antidepressants and to dependency treatment (at least).
Results: The search provided a scenario of a small number of drugs used for depression and drug dependency under active development, the highest development phases, the main mechanisms of action and the antidepressants used as antiaddictives. The patent landscape retrieved 2067 applied documents filed over the last 10 years, showing that China leads in the number of deposits of these drugs/compositions/methods to treat depression (with 1758 applications) made by Chinese Institutes and Universities, followed by the United States (291 applications) and Japan (193 applications). The patents’ search also allowed us to evaluate the documents which the claims addressed (at least) to the treatment of depression and as antiaddictive.
Conclusion: The analysis of under active development drugs presented those used as antidepressants and to the treatment of substances dependency in different stages of development, including the drugs in clinical studies. Indeed, the applications’ analysis on antidepressants has generated information about the technologies involved, as well as allowing the evaluation of the applications that are also promising for the treatment of addiction and depression.
ADD: Antidepressant Drug; CDDI: Cortellis Drug Discovery Intelligence; IND: Investigational New Drug; MAOIS: Monoamine Oxidase Inhibitor; SSRIs: Selective Serotonin Reuptake Inhibitor; SNRIs: Serotonin-Norepinephrine Reuptake Inhibitors; PNN: Patent Publication Number; TCAs: Tricyclic Antidepressant; UAD: Under Activity Development
Introduction
According to the World Health Organization (WHO) [1,2], there is an estimate that in low- and middle-income countries, 76-85% of subjects suffer with a mental disorder, with a lack of access to pharmacological treatment. The commom mental disorders, according to the International Organization, are the anxiety disorders and depressive ones, and both of them are prevalent in the population globally. Among the depressive diseases, depression commonly occurs, due to the fact that people are living more and the population is growing. During COVID-19 pandemic, depression increases face to its devastation aspects: millions of deaths all over the world, lack of social interaction and difficulty economic situation in all countries, mainly in low- and middle income ones [3]. The development of technologies/drugs/compositions for the treatment of depression (a condition that affects the global population) translates into more access to more individualized and effective treatments. This also translates into lower suicide rates and improved therapeutic options for addict subjects and greater treatment success.
Over 300 million people all over the world suffer from depression (4.4% of the population in the world). This suggests the increase of Antidepressant Drug (ADD) industry, due to the high consumption of these drugs. Not only for depression, but also, for other conditions, such as post-traumatics stress disorder, bipolar disorder, social anxiety disorder, major depressive disorder or chilhood enuresis have the ADDs as pharmacological treatment [4]. These drugs include: atypical agents, monoamine oxidase inhibitor (MAOIS), Tricyclic Antidepressant (TCAs), Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), selective serotonin reuptake inhibitor (SSRIs) (SNRIs and SSRI are the most used among elder adults) with different side effect profile and mechanism of action [5,6].
According to the document entitled “Abuse Principles of Drug Addiction Treatment: A Research-Based Guide” by the National Institute on Drug Abuse, there are drugs that can be included in the treatment of addiction (besides behavioral therapies or their combination), such as 1. Acamprosate, disulfiram and naltrexone (for alcohol dependence); 2. Naltrexone, buprenorphine and methadone (for opioid addiction) and 3. Bupropion and varenicline (tobacco addiction). Moreover, an important finding in the understanding of addicted subjects is that they offen suffer from depression (in general) and the ADDs and other psychoative medications (such as mood stabilizers, antipsychotic medications and anti-anxiety agents) are crucial for treatment effectivity [7].
The present study aims
1. To carry out a survey of the drugs under active development used to depression and dependency treatment (at least) for the treatment of addiction to different substances and 2. To evaluate, through the patent landscape1, the patent documents filed in 10 years related to this class of drugs in the context of technological scenario and to promote a discussion about drugs used as ADD and antiaddictive (at least).
Material and methods
Drugs Analysis from Cortellis Drug Discovery Inteligence (CDDI) Database (from Clarivate Analytics): CDDI Database focuses on drug and pharma development inteligence and provides pharmacological, chemical and biological data [9]. For the strategy search, the Database CDDI was choosen to retrieve ADDs Under Active Development (UAD) for the treatment of substance dependency, i.e., alcohol, cocaine and opiode dependency. For this purpose, the terms “depression” (including major depression, minor depression, pospartum depression, suicidal depression and treatment resistent depression) and “antidepressant” (inside the group of Psychopharmacologic Drugs) were used to the topic searches conditions associated to therapeutic group respectively in Advanced Search in CDDI Database. The mentioned strategy search was followed by the application of filters to obtain the ADDs to the treatment of substance dependency. It was not defined a period of time, due to the criteria is defined by the drugs UAD period. When a drug is UAD, this means (according to CDDI Database criteria) that it has been reported over the past 12-18 months (by Conferences, Peer Reviewed Journal Articles, Citation on the Company’s Website, Mention in Annual Reports, Clinical Trials Registers and Company Press Releases) in different development status, i.e., 1.preclinical testing; 2. Investigational New Drug (IND) filed; 3. Clinical; 4. Phases 0-III; 5. Pre-registered/registered and 6. Recommended approval [10].
Patent Landscape from Derwent Innovation Database (from Clarivate Analytics): Derwent Innovation is an analytic and patent research platform which provides access to scientific literature and patents globally [11]. Inside this database platform, it was developed a strategy search to assess technological information about ADDs. The strategy search consisted of searching for terms (antidepressant) or (depression and drug) or (depression and treatment) in the title and abstract fields, followed by the use of search codes A61P0025242 (International Patent Classification from the World International Property Organization) and B14-J01A1*3 (Manual Code from Derwent World Patents Index). The priority period of patent applications was 2010 – 2020. Here it will be analysed: 1 The most priority countries with the major number of applications; 2. The most institutions/universities/companies with the major number of applications; 3. The applications’ temporal evolution in the past ten years; 4. A discussion of filed documents about methods/compositions/drugs claimed as ADDs and antiaddictives (at least).
--------------------
1Patent landscape is the analysis of patent data. It can offer a tecnological scenario due to it can reveal scientific, business and technological trends. Moreover, it can provide data on patent validity and other informations related to the filed document [8].
2Code for antidepressants.
3Code for antidepressants.
Results
The developed strategy search, 163 drugs UAD were retrieved from Cortellis Drug Discovery Inteligence (CDDI) Database (from Clarivate Analytics). From them, 31 drugs used for substance dependency treatment, can be used to treat depression (at least). In From them: 14 drugs were used to the treatment of alcohol depencency; 12 to cocaine dependence and 2 to opioid dependency. As a drug can be used for more than one clinical condition (addiction), a total of 19 drugs used as antidepressants and alcohol, cocaine or opioid dependency were retrieved as showed in Figure 1. From the total of drugs, it was observed most of them are small molecules, which can have advantages (as ADDs), such as high potency, affinity and bioavalibility [12].
Another point to highlight is about the highest phase of development of these drugs (Figure 2). Here, it is possible to observe that most of the drugs were in clinical phase (Phase I: one drug and Phase II: five drugs). Two drugs were in pre-clinical phase and only one was pre-registered. It was also highlighted that only one drug was registered.
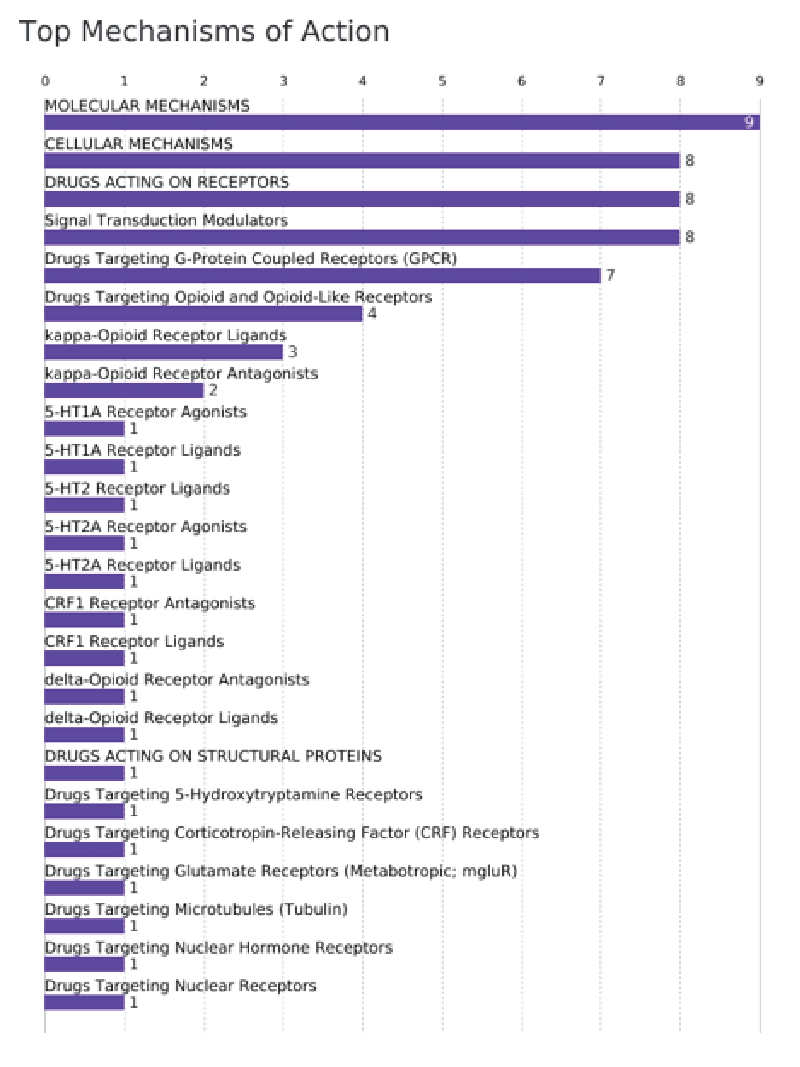
From the developed review, a key finding emerged: the top mechanism of action of these drugs, involving molecular, celular mechanisms and the action on receptors (Figure 3). Here, it is showed an overview of the main targets of molecule development from signal transduction modelators to specific receptors (5HT, dopanine and opioid ones).
It was also retrieved the ADDs (including) used to the treatment od substance dependency. The launched substances were excluded and only the substances UAD were showed (Table 1). Here, it is possible to identify in pre-registered phase and in clinical phase (phases I and II). The results’ analysis casted a new light on about the developmenn of the drugs (ADD and Antiaddictives): 1. A few number of substance for substance treatment and depression conditions; 2. Other conditions were also claimed by each substance and 3. Despite the few number of drugs, the companies (and its partnerships) are interested in the development of these therapeutic groups:
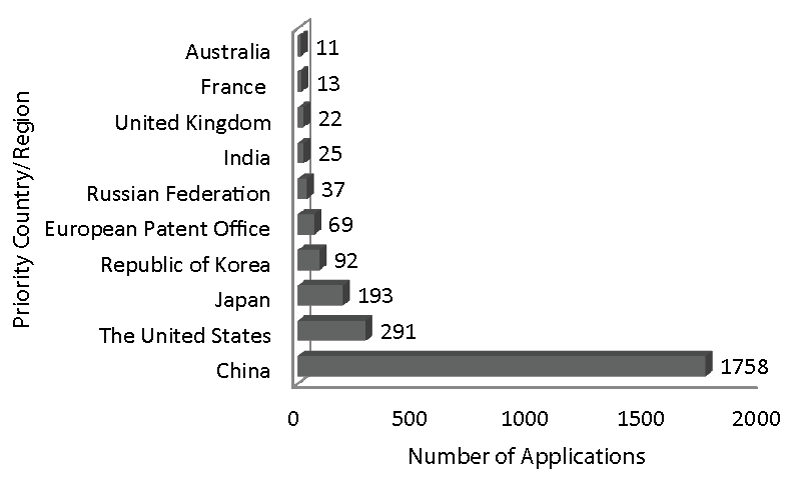
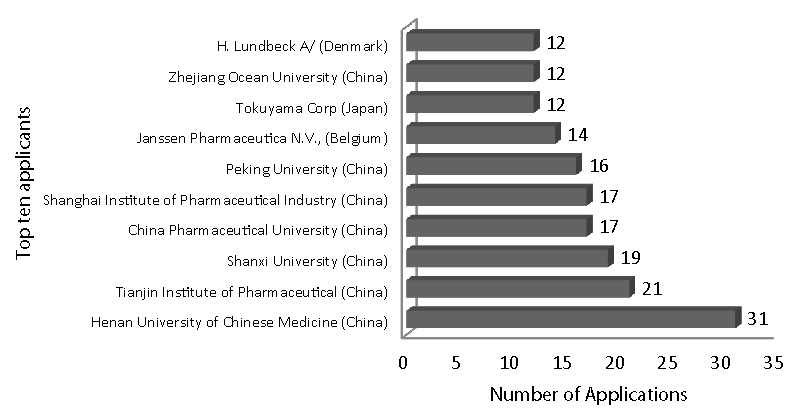
The applied search retrieved 2607 patent applied documents from Derwent Innovation Index in the period from 2010-2020. On average, 237 documents were applied per year, and in 2016 the most number of documents were applied (326). Regarding applicants (priority countries), China leads in the number of deposits (with 1758 applications) made by Chinese Institutes and Universities (as top five applicants: Henan University of Chinese Medicine (with 31 documents); Tianjin Institute of Pharmaceutical (21); Shanxi University (19); China Pharmaceutical University (17); Shanghai Institute of Pharmaceutical Industry (17) and Peking University (16).The top applicant was followed by the United States (291 applications) and Japan (193 applications) (Figures 4, 5).
Considering the use of drugs as ADD and antiaddictives (e.g), addiction disorders and depressive illness are highly prevalent [13]. Among the companies that stand out in the development of compounds to be used in pharmaceutical composition to treat depression and substance dependency (at least), there is the Japanese company TAISHO PHARMACEUTICAL CO LTD which claimed for a new carboxamide derivatives (PNN: JP2013249257A; JP2013249258A) or a new nitrogen-containing heterocyclic (PNN: WO2012036278A1).
The use of drugs to treatment can be also reported in the document applied by UMECRINE MOOD AB (from Japan). The drug can prevent central nervous system’s conditions and acts as GABA-A-receptor modulator, including the activity as antidepressant, antiaddictive and antialcoholic (PPN: JP05687287B2). SUN PHARMACEUTICAL INDUSTRIES LTD. (India) applied a method to treat conditions (including depression, alcohol related disorders, addiction of narcotic agents) which are susceptible to the drug baclofen (PPN: JP2013519726A). DUKE UNIVERSITY (US) applied methods and compositions to treat psychiatric and neurologic conditions (e.g. depression, addiction) by the administrationto the subject of “interfering molecule that inhibits tropomyosin-related kinase B (TrkB)-mediated activation of phospholipase C (PLC)-gamma-1” (PNN: US20110236371A1). NUTRICIA NV (Netherlands, from DANONE GROUP) applied a composition to prevent/treat disorders such as depression, abuse of alcohol/drugs, by the improvement of the fatty acid compositons of the membranes (PNN: CN104351796A).
ADD combined to cognitive behavioral can work best to depressed subjects [14]. EMORY UNIVERSITY (US) developed methods to improve behavioral therapy, by the administrion of a composition with oxytocin release agent. It acts as HT1A or 5-HT2A/C or melanocortin receptor agonist to be used as antidepressant, antiaddictive, neuroleptic (e.g.) (PNN: US20120108510A1).
The use of benzodiazepine antagonist, such as flumazenil is already described in literature for benzodiazepine dependence [15,16]. Here VERITA RESEARCH PTE LTD (from Singapore) applied a benzoadiazepine antagonist in a sustained release pharmaceutical composition with a biocompatible polymer. The composition is able to form gel-like depot and can be used to the treatment of diseases by different activities: antidepressant, antiaddictive, antialcoholic, etc (PNN: AU2011279557B2). Indeed, DUQUESNE UNIVERSITY (US) claimed by the psychostimulant antidepressant/antagonist composition which is “diphenyl piperidine derivative active agent with excipient or diluent”. It can be used to treat addiction in human/animal, psychostimulant dependence and to treat depression. The application document showed that the psychostimulant dependence is amphetamine or cocaine (PNN: US20120322824A1).
Among subjects addicted to alcohol or drugs, depression in common disease. Furthermore, the substance abuse is responsible for the intensification of feelings associated with depression, such as hopelessness, sadness and loneliness [17]. The applied document by UNITED STATES DEPARTMENT OF VETERANS AFFAIRS (US) and OREGON HEALTH & SCIENCE UNIVERSITY (US) (FORMERLY OREGON HEALTH SCIENCES UNIVERSITY) claimed by a neuropsychiatric or cognitive impairment (induced by substance addiction) where it is administered in a subject a major histocompatibility complex molecule (PNN: US20120195921A1).
Finally, the development of new compounds were observed: TAKEDA PHARMACEUTICAL COMPANY LIMITED (Japan) applied a new heterocyclic compound (mechanism of action: orexin receptor antagonist) to prevent/treat diseases such as drug dependence, depression, sleep disorder and anxiety (PNN: EP3287454B1). UNIV EAST CHINA NORMAL (China) developed a new isoxazole compound to preparate drugs with antidepressant and antiaddictive activity (e.g.) (PNN: CN107459510B). A kappa opioid receptor antagonist (tetrahydroisoquinolines) to treat nicotine, alcohol, methamphetamine and cocaine addiction, depression, schizophrenia and eating disorders were claimed by RESEARCH TRIANGLE INSTITUTE (NORTH CAROLINA) (US) (PNN: JP2019531260A). Indeed, the Swiss company F. HOFFMANN-LA ROCHE applied new annellated pyridine compounds to treat disorder as drug addiction, depression, psychotic disorders, etc. (PNN: BR112012032748A2).
Conclusion
The analysis of ADDs’ technological information generated data about the treatment of depression and substance dependency, such as the development of new compounds and with different targets. Although, companies stand out (TAISHO PHARMACEUTICAL CO LTD) on drugs’ research and development and a promising number of data involving ADDs were observed, there were few results also involving the treatment of addiction, which demonstrates the need for more research, development and innovation to promote more therapeutic options and more access to medicines.
- World Health Organization. Depression. 2021. Link: https://bit.ly/3uX6DqV
- World Health Organization (2017) Depression and Other Common Mental Disorders Global Health Estimates. Link: https://bit.ly/3BqqLEn
- Abbott A (2021) Covid’s mental-health toll: scientists track surge in depression. Nature 590. Link: https://go.nature.com/3lrEIfQ
- Businesswire (2021) Global Antidepressants Market Report 2021: COVID-19 Causes a Surge in Demand for Antidepressant Drugs as Mental Health Problems Rise - ResearchAndMarkets.com. Business Wire. Link: https://bwnews.pr/3Ao6cqD
- Ogbru A (2021) The Comprehensive List of Antidepressant Medications & Drug Class. 2021 Link: https://bit.ly/3AqeDBY
- Haddad YK, Luo F, Bergen G, Legha JK, Atherly A (2021) Special Report from the CDC Antidepressant subclass use and fall risk in community-dwelling older Americans. J Safety Res 76: 332-340. Link: https://bit.ly/2WVdkgC
- National Institute on Drug Abuse (2018) What is drug addiction treatment? | National Institute on Drug Abuse (NIDA). Link: https://bit.ly/3iMHsT3
- Queen’s University Library (2021) Patent Landscape Analysis - Patents and Designs - Research Guides at Queen’s University. Link: https://bit.ly/3iLbx5w
- Clarivate (2021) Introducing Cortellis Drug Discovery Intelligence - Cortellis. Introd Cortellis Drug Intell. 2021 Link: https://bit.ly/3BsTeJN
- Clarivate (2021) Cortellis Drug Discovery Intelligence Glossary & Help File. Link: https://bit.ly/2WZIbsy
- Clarivate (2021) Derwent Innovation - Derwent. new Derwent Innov is here. Link: https://bit.ly/3v8UW0F
- Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, et al. (2005) Innovative Approaches for the Development of Antidepressant Drugs: Current and Future Strategies. NeuroRx 2: 590. Link: https://bit.ly/3Fw0t5R
- DeVido JJ, Weiss RD (2012) Treatment of the Depressed Alcoholic Patient. Curr Psychiatry Rep 14: 610-618. Link: https://bit.ly/3FpzrNL
- American Addiction Centers (2021) A Combination of Cognitive Behavioral Therapy & Antidepressant Medication Works Best for Depressed Adolescents - Depression Resources, Education About Depression and Unipolar Depression. Link: https://bit.ly/3Fyncyc
- Hood SD, Norman A, Hince DA, Melichar JK, Hulse GK (2014) Benzodiazepine dependence and its treatment with low dose flumazenil. Br J Clin Pharmacol 77: 285-294. Link: https://bit.ly/3AslgDE
- PW M, JW D, Burkhart KK, Waskin JA, Hieger MA, et al. (2014) Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 10: 126-132. Link: https://bit.ly/2X1deUU
- Addiction Center (2021) Depression and Substance Abuse. Link: https://bit.ly/3Fx9N9S
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
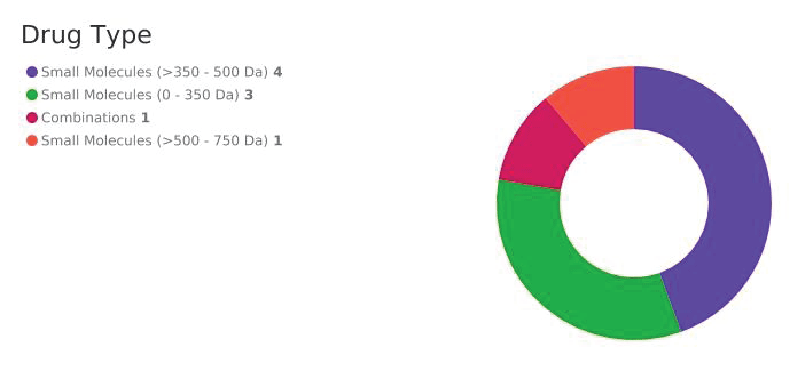
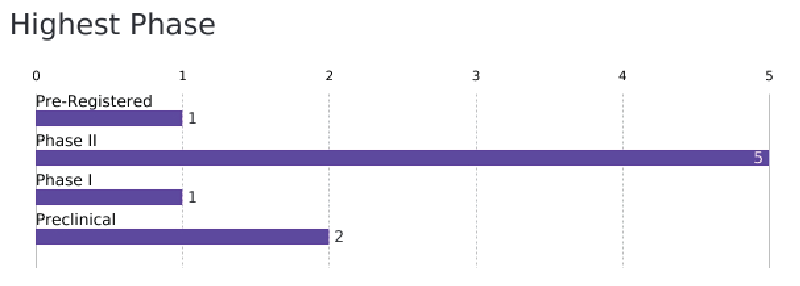


 Save to Mendeley
Save to Mendeley
